Presentation
International Hospital Diabetes Meeting
Date
May 2015
Authors
Melanie Mabrey, Joseph Aloi, Paul Chidester, Amy Henderson, Raymie McFarland, Robby Booth, Andrew Rhinehart
Objective
The American Diabetes Association guidelines recommend a basal bolus correction insulin regimen as the preferred method of treatment for non-critically ill hospitalized patients. The targets for pre-meal blood glucose (BG) of <140 mg/dL and random BG of <180 mg/dL are well defined. However, achieving these targets safely, without hypoglycemia, is challenging. In this stud we evaluated BG control before and after Glucommander (GM) subcutaneous treatment for patients outside of the intensive care unit.
Methods
- 2,240 non-ICU patients were treated with subcutaneous insulin therapy directed by the provider.
- Insulin therapy was then directed by Glucommander Subcutaneous (GM SubQ).
- 9 different hospitals in the Sentara Healthcare.
- Analysis: Before Glucommander (BGM) and During GM (DGM) for all patients. After GM (AGM) was included if GM was stopped prior to discharge.
- Target BG was 140-180 mg/dL for all groups.
- Safety was evaluated by average BG and BGs <70 mg/dL.
Results
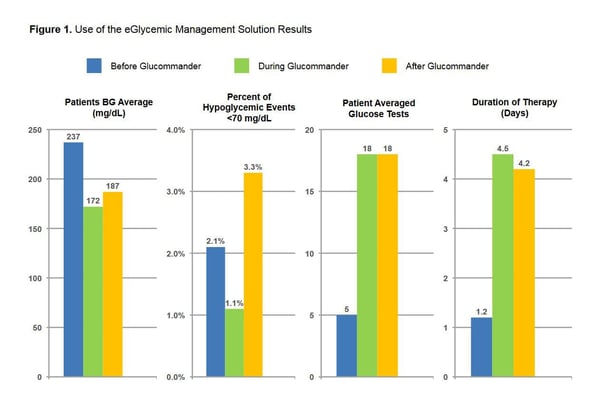
- BGM BG average was 237 mg/dL.
- DGM BG average was 172 mg/dL.
- AGM BG average was 187 mg/dL.
- Hypoglycemia events <70 mg/dL, 2.1% BGM, 1.1% DGM and 3.3% AGM treatment. Patients averaged 5 glucose tests before Glucommander was initiated. Patients had an average of 18 glucose tests after treatment with GM. Duration of therapy BGM was 1.2 days, DGM 4.5 days, and AGM was 4.2 days.
Conclusion
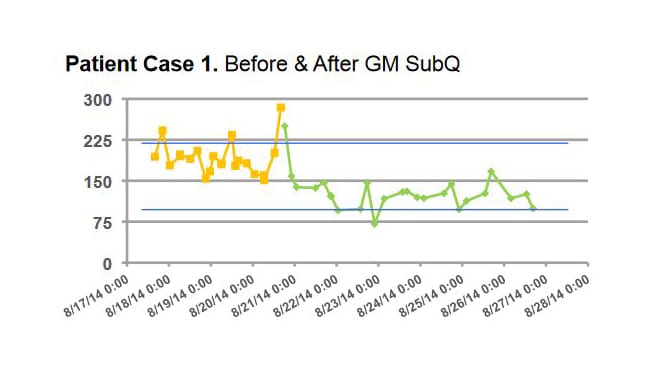
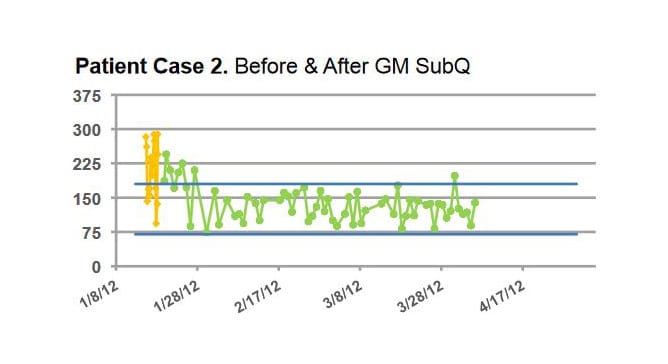
Patients using GM SubQ achieved improved glycemic control with lower incidence of hypoglycemia (<70 mg/dL) compared to both Before and After GM management. These results suggest that GM can safely maintain glucose control with less hypoglycemia than standard basal bolus treatment.