Publication
Current Diabetes Reports
Date
Accepted 26 March 2023
Authors
Kelly Engle¹, Grace Bacani², Curtiss B. Cook3, Gregory A. Maynard4, Jordan Messler5, Kristen Kulasa¹.
¹UCSD Division of Endocrinology, San Diego, CA, USA, ²UCSD Nursing Development, Education and Research, San Diego, CA, USA, USA ³Mayo Clinic Arizona Division of Endocrinology, Phoenix, AZ, USA 4UC Davis Chief Quality Officer, Sacramento, CA, USA,5 Glytec Chief Medical Officer, Largo, FL, USA
Abstract
Purpose of Review Inpatient glucose data analysis, or glucometrics, has developed alongside the growing emphasis on glycemic control in the hospital. Shortcomings in the initial capabilities for glucometrics have pushed advancements in defining meaningful units of measurement and methods for capturing glucose data. This review addresses the growth in glucometrics and ends with its promising new state.
Recent Findings Standardization, allowing for benchmarking and purposeful comparison, has been a goal of the field. The National Quality Foundation glycemic measures and recently enacted Center for Medicare and Medicaid Services (CMS) electronic quality measures for hypo- and hyperglycemia have allowed for improved integration and consistency.
Summary Prior systems have culminated in an upcoming measure from the Center for Disease Control and Prevention’s National Healthcare Safety Network. It is poised to create a new gold standard for glucometrics by expanding and refining the CMS metrics, which should empower both local improvement and benchmarking as the program matures.
Keywords Diabetes · Hospital · Hypoglycemia · Hyperglycemia · Metrics · Glucometrics
Introduction
Inpatient dysglycemia is associated with poorer patient outcomes and higher financial costs. As the prevalence of diabetes and hyperglycemia in the hospital rise, there has been growing interest in addressing inpatient glucose control [1–4]. Key to improving inpatient glycemic control in any institution is the use of standardized glucose performance metrics or “glucometrics.” Glucometrics have lacked national definitions, clarity, and standards, but efforts from national organizations have helped move this field forward. The National Quality Foundation (NQF) hypoglycemia and hyperglycemia measures were an important step and led to the current implementation of the Center for Medicare and Medicaid Services (CMS) electronic clinical quality measures (eCQMs) that will ideally help raise awareness and attention to the important problem of inpatient dysglycemia. The future state of inpatient blood glucose analysis is evolving with National Healthcare Safety Network (NHSN) measures and a reporting system currently being developed which could establish a new gold standard for glucometrics. The goal of this review is to discuss glucometrics, including its definition and scope as well as its role in patient care and safety in the hospital.
Glucometrics
Glucometrics is the “systematic analysis of inpatient blood glucose data” and is used to track glycemic control over time [5]. The rationale to use glucometrics is manifold; it includes allowing for the assessment of quality improvement (QI) projects and to justify the provision of resources for such initiatives including assessing differences in control between hospital units, prioritizing QI efforts, reassuring staff of safety and effectiveness of protocols, and to gauge the impact of efforts with a balanced scorecard. Also, while a consensus on how to define and report them is yet to be established, glucometrics allows for data comparison among patient care units and hospitals that report data similarly [6–8]. Moreover, interest in inpatient glycemic control is evolving now to include pay for performance models. Hospital readmission reduction programs, non-payment for hospital-acquired conditions programs, and financial incentive for quality improvement, such as the Quality Payment Model from the Center for Medicare and Medicaid Services that will be addressed in next sections, are potential areas of impact [9]. Thus, having a means to track performance on glycemic measures may become a financial essential for hospitals [10, 11].
Case Selection
Integral to developing a systematic analysis of blood glucose data is defining the population, values, and methods to be included and excluded. Investigations of inpatient glycemic management helped reveal the most clinically meaningful and practical measures. In terms of target population, patients with diabetic ketoacidosis (DKA) or hyperosmolar hyperglycemic state, pediatric patients, or patients who are pregnant should preferably be separated out and analyzed independently as targets and management protocols are distinct. Comfort care patients should be excluded in data analysis if possible. Other populations often excluded, to allow for meaningful interpretation of inpatient glucose data, are patients with less than one day in hospital or less than 4–5 total glucose readings. Glucose values from the first day of hospitalization, given this may be more reflective of home glycemic management and other factors that do not reflect inpatient management, and values after day 14 of a hospital stay may be considered for exclusion, although these values remain a part of some current benchmarking sources [11]. Repeat glucose values are often performed after a hypoglycemic excursion, and these readings should be scrutinized with the initial low reading discounted if the reflex reading is normal. A hypoglycemic “event” needs to be clearly defined during data extraction so as not to include repeated low measurements within the same occurrence. Hypoglycemia in patients not on an antihyperglycemic agent, which can occur in critical illness or liver failure, should also not be a part of this analysis if possible. In reality, addressing all of these exclusion criteria is arduous and requires a robust data analysis. It is difficult to compare data sets without knowing which of these exclusions have been applied, and therefore, it should be clearly stated when discussing glucometrics data locally, and when comparing to other data sets. This issue makes the case for national standardized metrics to allow benchmarking across systems and comparison over time.
When discussing glucose values, point-of-care (POC) testing of capillary blood glucose (BG) is a practical, real time method of measurement commonly used in most hospitals and therefore the standard for inclusion in analysis. However, especially in critical care settings, there are limitations to POC BG accuracy and inclusion of additional glucose measurement methods may be appropriate. With benefits in glucose trend detection and hypoglycemia prevention, continuous glucose monitoring (CGM) may have potential future consideration in the field of glucometrics [4, 12]. However, at this time, CGM is not included in standard analyses as more investigation is needed to validate its accuracy, use, and safety.
Units of Analyses
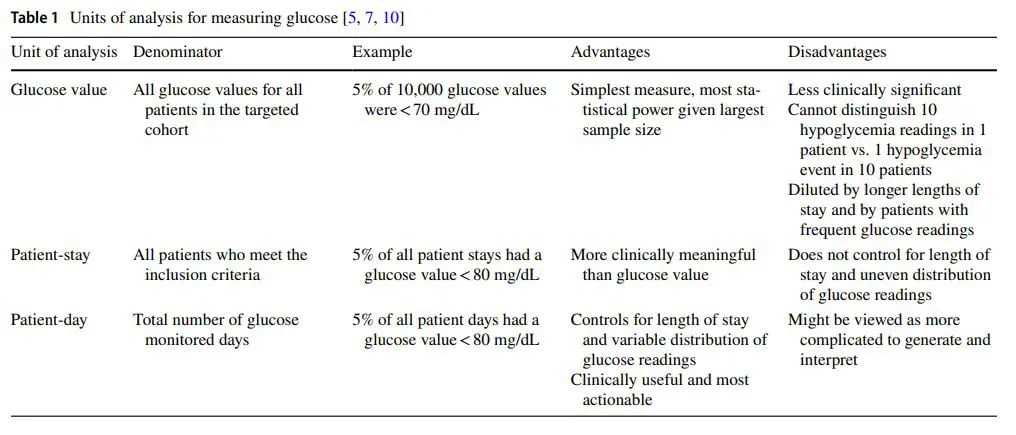
There are different units of analyses used in glucometrics, each with their own advantages and disadvantages. Table 1 describes the most common units of analysis, the denominators used in calculations, and the advantages and disadvantages of each.
The following example (Fig. 1) uses all three units of analysis to determine the rate of hypoglycemia, demonstrating the different, but complementary information that each method provides.
In one month, 1,483 glucose measurements were obtained from 112 patients representing 447 monitored patient days. With hypoglycemia defned as BG<54 mg/dL, the results showed the following: 28 of 1,483 measurements (1.9%) were hypoglycemic, 8 of 112 patients (7.1%) had at least one hypoglycemic episode over their stay, and 18 of 447 monitored days (4.0%) had at least one hypoglycemic episode.
Different methods of analysis have been used to try to best capture and summarize glucose values in evaluating glycemic control. The hyperglycemic index, which is a method of analysis assessing hyperglycemic values over length of stay without inclusion of hypoglycemia, has not found benefit versus use of mean glucose [7].
More Description of Metrics
Table 2 provides information on additional metrics building on glucose values, patient days, and patient stays units and examples of hypoglycemia management analysis. Some of these are outcome metrics, and others, related to hypoglycemia are process metrics. A combination of metric types are often useful to measure impact of QI efforts on both the long and short term. Evaluating glycemic control in non-critical care versus critical care settings should be done separately, as several factors make comparison between them difficult. For one, glycemic targets in these settings are at different levels and varying consistency. In non-critical care settings, the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists (AACE) recommend to target fasting glucose < 140 mg/dL and random glucose <180 mg/dL while the Endocrine Society recommends to maintain glucose in the range of 100 to 180 mg/dL [11, 12]. More than differing targets, variability in the definition of hyperglycemia and severe hyperglycemia also remains. In critical care settings, inconsistencies in recommendations are also present. After the NICE SUGAR trial found increased mortality and hypoglycemia in “very tight” versus moderate glycemic control, societies revised guidelines to recommend initiation of insulin therapy for glucose > / = 180 mg/dL and to maintain levels between 140 to 180 mg/dL in critically ill patients. The Society of Critical Care Medicine, however, continues to recommend maintaining glucoses between 100 to 150 mg/dL but emphasizing hypoglycemia prevention [11]. Another factor that obfuscates the comparison between non-critical care and critical care settings is the differing methods to achieve glycemic control in these settings. Scheduled subcutaneous insulin administration is the preferred method in the non-critically ill settings, while insulin infusion is recommended for most critically ill patients [11]. With these different methods come different frequencies of glucose checks, with insulin infusion far outnumbering subcutaneous insulin in the opportunities to adjust insulin therapy to reach or maintain glycemic control.
Standardization: a Work in Progress
As noted above, despite glucometrics having a higher than ever visibility, there has been a lack of consensus as several organizations propose different types of measures and even how to determine and calculate them. The absence of consensus has been a key barrier to the development of a systematic analysis of glucose data. Standardization is necessary to be able to compare systems internally over time, such as a floor unit after a quality intervention made, and organizations externally to each other. While there has been debate on practical definitions, goal glycemic targets, and meaningful methods of data analysis, there is now an evolving consensus in a number of these areas. Hypoglycemia can be defined in multiple ways as no one cut-of serves all purposes. The ADA currently categorizes hypoglycemia into levels with level 1 being BG of less than 70 mg/dL, level 2 BG less than 54 mg/dL, and level 3 as characterized not by a value but by mental status change or an episode necessitating outside assistance [4]. A variety of cut-of points as well as use of both patient-day and patient-stay unit of analyses is helpful for improvement efforts as BG of 300 mg/dL is a safety issue and taking some action if BG is persistently>180 mg/dL is desirable.
Historical Regulatory Measures
Regulatory measures for glycemia were initially introduced in 2008, as part of the Surgical Care Improvement Project (SCIP) as well as in the Inpatient Prospective Payment System (IPPS). SCIP’s initial goals included reduction of surgical complications. The SCIP INF 4 measure was a glycemic measure introduced to help meet this goal, with the aim of a 0600 AM post-operative serum glucose of less than 200 mg/dL in cardiovascular surgical patients. This measure, and all the SCIP measures, were discontinued in 2015 [13] as sites achieved this goal successfully without a clear relation to improvements in outcomes [14]. The 2008 IPPS final rule included glycemic conditions that were at risk for hospital acquired condition (HAC) payment reduction. Hospital acquired DKA or hypoglycemia coma, for instance, was included in this CMS rule.
Attempts to Craft Better Metrics
Given the significant complexity and expense of developing reproducible and comparable local measures of glycemic control, multiple external sources have established their own reporting systems. These external sources include the Remote Automated Laboratory Systems (RALS) application, the Yale Web-based system (no longer in service), and the Society of Hospital Medicine (SHM) Glucometrics Web-based system. While these sources differ in their exact metrics and reports and have varying methods of data upload and ability to provide benchmarking, they have helped move the field forward by establishing a systematic approach to accessible glucometrics that institutions can use to help support improvement efforts.
Vizient
The Vizient (Formerly University of HealthSystem Consortium) clinical database provides a variety of analytics, including a hypoglycemic metric. With over 1000 hospitals, enrolled sites can comparatively evaluate themselves for hypoglycemia. While Vizient’s exact measures are proprietary and not available for publication, their inclusions and details are generally not part of other data sets, which limits the ability to compare to other similarly reported metrics, from RALS data or SHM for instance.
NQF
In 2014, the National Quality Foundation (NQF) designed a hypoglycemia measure (NQF 2363) and hyperglycemia measure (NQF 2362), which were endorsed in Spring 2019 after being tested and validated at 6 hospitals [15, 16••]. This initial measure evaluated hypoglycemia events as patient-days and allowed for repeat events if they occurred more than 20 h later. Ultimately, these measures were not introduced due to challenges in the query for abstracting the data from the electronic health record (EHR), costs related to data extraction, and lack of standards. Iterative improvements in the measure allow for easier electronic capture from the EHR and evolved into NQF 3503e for severe hypoglycemia and 3533e for severe hyperglycemia [17].
CMS eCQMs
In the state of wide variation, limited improvement over time, and lack of clarity of metrics, CMS finalized two new metrics based on these NQF measures with the final rule announced August 13, 2021. The aims of the metrics are to raise awareness, reduce variation, and spur improvements in the quality of glycemia management by driving reduction in preventable harm.
The eCQMs are part of the Hospital Inpatient Quality Reporting Program (IQR) and categorized as Preventable Healthcare Harm. In this program, CMS collects data electronically from hospitals with reported data publicly displayed, available on the Hospital Care Compare website [18].
The reporting period for these measures begins January 2023, with payment determination beginning in the CMS fiscal year of 2025 (October). This is a pay for reporting program, different than other pay for performance programs. Hospitals will choose 4 of the 11 eCQMs to report, one will be a required opioid related measure, and sites can choose both, one or none of the glycemia measures to report. If hospitals do not report eCQMs, they face a payment reduction of one quarter of their annual payment rate update. Table 3 provides details on the severe hypoglycemia and hyperglycemia measures.
Both will include only designated inpatient stays, though the event could have occurred in the emergency room or during an observation period of an inpatient stay.
As seen with other regulatory measures, such as sepsis bundle [19], or congestive heart failure [20] regulatory measures can have an immediate impact on the delivery of care. One major limitation of these measures to drive improvement will be the availability of results long after the events have occurred: in October of the year after reporting begins. In addition, hypoglycemia and hyperglycemia are balancing measures, meaning hyperglycemia reduction at the expense of additional hypoglycemia would not deliver the intended results. Hence, being aware of both measures is important for improvement. Ideally, both measures are tracked together. In addition, these measures do not specify results based on location, such as intensive care unit (ICU) or non-ICU setting, which, as described earlier, ICU and non-ICU locations have different processes (intravenous versus subcutaneous insulin management, for instance) and may have differing results of hypoglycemia and hyperglycemia.
NHSN/CDC Measures: an Emerging Gold Standard
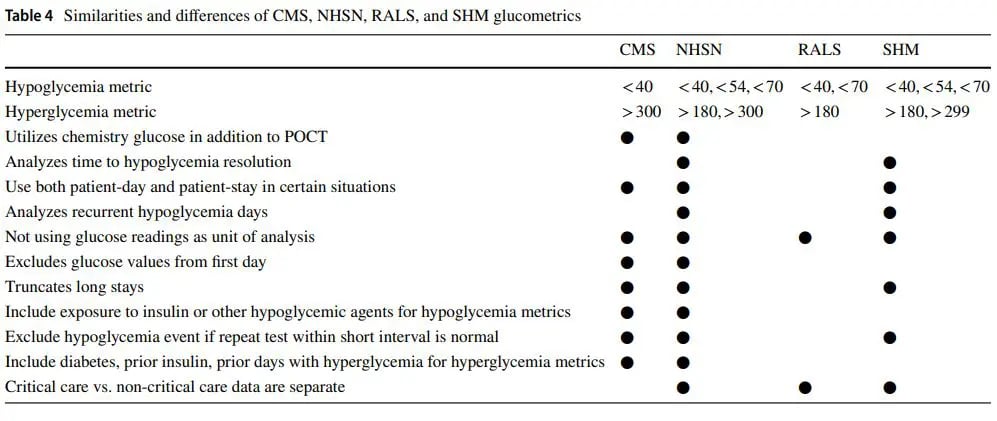
The Center for Disease Control and Prevention’s National Healthcare Safety Network (NHSN) is the largest healthcare event tracking system in the US. NHSN is creating a glycemic control module that will build on the strengths of the new CMS eCQM measures, while augmenting these measures in ways that will overcome many of their limitations [21•]. The NHSN measures have the goals of being timelier, more comprehensive, and more granular, with the capacity to drive local improvement efforts that the eCQMs lack. More timely measures can help hospitals understand the impact of introduced quality improvements. In addition, process metrics such as recurrent hypoglycemia and resolution time of hypoglycemia assist sites in evaluating the impact of process improvements. Hospitals will also be able to see more metric results, such as stay metrics, as well as metrics depicting the percent BGs in range and day weighted mean BG for specified cohorts. More details on the specifics abilities of the early, current, and developing glucometrics systems are presented below in Table 4.
Strengths and Weaknesses of Current State
Despite years of discussion, we are still reaching toward achieving “glucometric harmonization.” As noted above, quality focused organizations are still proposing metrics utilizing different inclusions, exclusions, numerators, denominators, and glucose thresholds to be analyzed. However, there is emerging consensus on many factors including need for different cut-of values for hypo and hyperglycemia, associating hypoglycemia with prior exposure to hypoglycemia inducing agent when feasible, excluding hypoglycemia when rapid repeat values are in normal range, and need to monitor recurrent hypoglycemic days. With the addition of glycemic measures to the CMS eCQM system, there is now a start to accountability in reporting with financial incentive.
Glucometrics should not exist in a vacuum. The reason inpatient glucose is relevant is because of the association of dysglycemia with poorer inpatient outcomes. Therefore, reporting glucometrics in relation to clinical outcomes, such as surgical site infections or sepsis, would be more informative. Because insulin therapy in the hospital is one of the primary means of controlling blood glucose, reporting glucometrics in association with insulin metrics (also known as insulinometrics) could be more informative in how providers are managing hyperglycemia [6]. Finally, understanding hospital policies and procedures in relation to glucose management (e.g., prevention and treatment of hypoglycemia) in relationship to glucose control could lead to dissemination of best practices. SHM and NHSN measures on time to resolution of hypoglycemia were implemented to address this important point.
The final pathway to glucometric harmonization is data source harmonization. If POC glucose data is to be used in benchmarking initiatives, then measurements should be performed on the same instrument type. Given the difficulty of attaining this in reality, data sources are often merged. While aggregating blood glucose and POC glucose values together has its challenges in analysis, potential benefits include not missing a significant hyper or hypoglycemic event. This remains an area without consensus.
Future State
The development of eCQMs allows capture of data from the EHR to enable integration of real time patient care and collection of glucose data. The CMS eCQM and subsequent NHSN modules have created standardization in the field, such as on levels of unacceptably low BG and severe hyperglycemia, allowing for improved benchmarking and quality improvement. The topic of specific glucose targets will likely be ongoing as there has been evidence for varying intensities of target glucose ranges depending on the person’s pre-existing glycemic background and hospital circumstance [22•]. However, SHM and NHSN allow for separation of glucose data by units and care types which is a step toward tackling this issue. With the struggles of sites lacking the informatics resources to do this work of data collection and analysis, NSHN is stepping in to fill that need and may ultimately be where sites go to create glucometrics.
Conclusion
The importance of inpatient glucose data analysis allowing for improved glycemic management has been emphasized by many studies, professional societies, and now national entities. While differences have existed for years in regard to glucose targets and methods of analysis, there is developing consensus in this area. Patient-day and patient-stay metrics have been found to be clinically meaningful. RALS system and SHM center provided the start to benchmarking. A major development in the standardization of glucometrics, and highlighting the significance of its use, came from NQF hyperglycemia and hypoglycemia measures which led to the implementation of glycemic measures in the 2022 CMS eCQM program. The emerging NHSN modules aim to take momentum from the recent eCQM measures and expand with benchmarking and real time monitoring of severe hypoglycemia and hyperglycemia in the hospital. We anticipate inclusion of these metrics in future pay for reporting and pay for performance programs, as well as playing a role in the hospital accreditation process. The progress in the field of glucometrics is important in continuing to improve the health and outcomes of the large population of people with diabetes and hyperglycemia in the hospital.
Competing Interests Jordan Messler is a paid employee of Glytec, Chief Medical Officer. Gregory A Maynard acts as a subject matter expert for SHM and NHSN Glucometrics. The other authors declare no competing interests.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- Lansang CM, Umpierrez GE. Inpatient hyperglycemia management: a practical review for primary medical and surgical teams. Cleve Clin J Med. 2016;83:S34–43. https://doi.org/10.3949/ccjm.83.s1.06.
- Ramos M, Khalpey Z, Lipsitz S, Steinberg J, Panizales MT, Zinner M, Rogers SO. Relationship of perioperative hyperglycemia and postoperative infections in patients who undergo general and vascular surgery. Ann Surg. 2008;248:585–91. https://doi.org/10.1097/SLA.0b013e31818990d1.
- Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail-Beigi F, Kirkman MS, Umpierrez GE. American Association of Clinical Endocrinologist and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32:1119–31. https://doi.org/10.2337/dc09-9029.
- American Diabetes Association Professional Practice Committee; American Diabetes Association Professional Practice Committee: Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, Freeman R, Green J, Huang E, Isaacs D, Kahan S, Leon J, Lyons SK, Peters AL, Prahalad P, Reusch JEB, Young-Hyman D, Das S, Kosiborod M. 16. Diabetes care in the hospital: standards of medical care in diabetes-2022. Diabetes Care. 2022 Jan
1;45(Suppl 1):S244-S253. https://doi.org/10.2337/dc22-S016. - Goldberg PA, Bozzo JE, Thomas PG, Mesmer MM, Sakharova OV, Radford MJ, Inzucchi SE. “Glucometrics”–assessing the quality of inpatient glucose management. Diabetes Technol Ther. 2006;8(5):560–9. https://doi.org/10.1089/dia.2006.8.560.
- Thompson BM, Cook CB. Glucometrics and insulinometrics. Curr Diab Rep. 2017;17(12):121. https://doi.org/10.1007/ s11892-017-0964-2.
- Schnipper JL, Magee M, Larsen K, Inzucchi SE, Maynard G. Society of Hospital Medicine Glycemic Control Task Force Society of Hospital Medicine Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control eforts. J Hosp Med. 2008;3(5 Suppl):66–75. https://doi.org/10.1002/jhm.356.
- Maynard G, Schnipper JL, Messler J, Ramos P, Kulasa K, Nolan A, Rogers K. Design and implementation of a web-based reporting and benchmarking center for inpatient glucometrics. J Diabetes Sci Technol. 2014;8(4):630–40. https://doi.org/10.1177/1932296814532237.
- Mathes T, Pieper D, Morche J, Polus S, Jaschinski T, Eikermann M. Pay for performance for hospitals. Cochrane Database Syst Rev. 2019;7(7):CD011156. https://doi.org/10.1002/14651858. CD011156.pub2.
- Cook CB, Wellik KE, Kongable GL, Shu J. Assessing inpatient glycemic control: what are the next steps? J Diabetes Sci Technol. 2012;6(2):421–7. https://doi.org/10.1177/193229681200600230.
- Maynard G, Berg K, Kulasa K, O’Malley C, Rogers KM. The glycemic control implementation guide: improving glycemic control, preventing hypoglycemia and optimizing care of the inpatient with hyperglycemia and diabetes (2nd ed.). Society of Hospital Medicine. 2015. https://www.hospitalmedicine.org/globalassets/clinical-topics/clinical-pdf/gcmi-guide-m4.pdf. Accessed 26 Sep 2022
- Korytkowski MT, Muniyappa R, Antinori-Lent K, Donihi AC, Drincic AT, Hirsch IB, Luger A, McDonnell ME, Murad MH, Nielsen C, Pegg C, Rushakof RJ, Santesso N, Umpierrez GE. Management of hyperglycemia in hospitalized adult patients in non-critical care settings: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2022;107(8):2101–28. https://doi.org/10.1210/clinem/dgac278.
- Branch-Elliman W, Elwy AR, Lamkin RL, Shin M, Engle RL, Colborn K, Rove J, Pendergast J, Hederstedt K, Hawn M, Mull HJ. Assessing the sustainability of compliance with surgical site infection prophylaxis after discontinuation of mandatory active reporting: study protocol. Implement Sci Commun. 2022;3(1):47. https://doi.org/10.1186/s43058-022-00288-0.
- LaPar DJ, Isbell JM, Kern JA, Ailawadi G, Kron IL. Surgical Care Improvement Project measure for postoperative glucose control should not be used as a measure of quality after cardiac surgery. J Thorac Cardiovasc Surg. 2014;147(3):1041–8. https://doi.org/10.1016/j.jtcvs.2013.11.028.
- Measures inventory tool. Centers for Medicare & Medicaid Services. http://cmit.cms.gov/cmit/#/. Accessed 30 Sep 2022.
- •• Santos CAQ, Conover C, Shehab N, Geller AI, Guerra YS, et al. Electronic measurement of a clinical quality measure for inpatient hypoglycemia events: a multicenter validation study. Med Care. 2020;58(10):927–33. https://doi.org/10.1097/MLR. 0000000000001398. The study assessed the National Quality Forum glycemic criteria and validated the ability to identify inpatient hypoglycemia using electronic queries. This development paved the way the subsequent Center for Medicare and Medicaid Services electronic measures.
- Electronic clinical quality improvement resource center. Centers for Medicare & Medicaid Services. https://ecqi.healthit.gov. Accessed 26 Sep 2022
- Hospital compare. Centers for Medicare & Medicaid Services. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/HospitalCompare. Accessed 30 Sep 2022
- Pakyz AL, Orndahl CM, Johns A, Harless DW, Morgan DJ, Bearman G, Hohmann SF, Stevens MP. Impact of the centers for Medicare and Medicaid services sepsis core measure on antibiotic use. Clin Infect Dis. 2021;72(4):556–65. https://doi.org/10.1093/cid/ciaa456.
- Desai NR, Ross JS, Kwon JY, Herrin J, Dharmarajan K, Bernheim SM, Krumholz HM, Horwitz LI. Association between hospital penalty status under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA. 2016;316(24):2647–56. https://doi.org/10.1001/jama.2016.18533.
- • NHSN Reporting: Adverse Drug Events – Glycemic Control 0.1.0 – CI Build. HL7 International – Public Health 2022 Jan 10. http://build.fhir.org/ig/HL7/fhir-nhsn-ade-ig/ The National Healthcare Safety Network is introducing hypoglycemia and hyperglycemia modules to address medication safety, patient safety, and quality improvement in inpatient glucose management.
- • Krinsley JS, Preiser JC, Hirsch IB. Safety and efcacy of personalized glycemic control in critically ill patients: a 2-year before and after interventional trial. Endocr Pract. 2017;23(3):318–30. https://doi.org/10.4158/EP161532.OR. Outcomes in patients based on glycosylated hemoglobin and varying glycemic goal ranges in the hospital were analyzed. This study points to future development in more personalized glucose targets.