Publication
Journal of Diabetes Science and Technology
Date
September 28, 2020
Authors
Rodolfo J. Galindo, MD, FACE1 , Guillermo E. Umpierrez, MD, CDE1, Robert J. Rushakoff, MD2, Ananda Basu, MD, FRCP3, Suzanne Lohnes, MA, RN, CDCES, CPT4, James H. Nichols, PhD, DABCC, FAACC5 , Elias K. Spanakis, MD6,7, Juan Espinoza, MD, FAAP8, Nadine E. Palermo, DO9, Dessa Garnett Awadjie, MSN, FNP-BC, BC-ADM, CDCES10, Leigh Bak, MSN, APRN, ACNS-BC, CDCES11, Bruce Buckingham, MD12, Curtiss B. Cook, MD13, Guido Freckmann, MD14, Lutz Heinemann, PhD15, Roman Hovorka, PhD, FMedSci16, Nestoras Mathioudakis, MD, MHS17, Tonya Newman, JD18, David N. O’Neal, MD, FRACP19, Michaela Rickert, MS, PA-C, RDN, CDE20, David B. Sacks, MB, ChB, FACP, FRCPath21 , Jane Jeffrie Seley, DNP, MPH, MSN, GNP, RN, BC-ADM, CDCES, CDTC, FADCES, FAAN22, Amisha Wallia, MD, MS23, Trisha Shang, BA24, Jennifer Y. Zhang, BA24, Julia Han, BA24, and David C. Klonoff, MD, FACP, FRCP (Edin), Fellow AIMBE25
ABSTRACT
Introduction
Continuous glucose monitors (CGMs) are becoming an important technology for improving glycemic outcomes in diabetes. The opportunity for a patient (or by way of wireless communication, a caregiver, or relative) to see real-time glucose concentrations tested automatically and continuously is transforming the practice of diabetes care. Recent generations of these devices offer improved accuracy, smaller form factors, extended sensor life, and new data presentation software for translating data into increasingly useful metrics on various mobile platforms. Some new factory-calibrated CGMs have eliminated the need for finger-stick blood glucose (BG) testing by users (except at certain times per individual product instructions, such as soon after insertion, when there appear to be errors or no readings at all, when the CGM value does not match how the patient feels, or when an icon indicates the need for testing BG).
CGMs for monitoring glucose concentrations and automated insulin dosing (AID) systems, which contain a CGM controlling a continuous subcutaneous insulin infusion (CSII) system (also known as an insulin pump), are cleared (class II) or approved (class III) by the United States Food and Drug Administration (FDA) for home use (by prescription) by people who have diabetes. However, many clinicians believe that CGMs have the potential to be utilized by hospitalized patients in a variety of situations.
Escalating interest in utilizing CGMs and AID systems in a hospital setting has resulted in a need for guidance on the continuation of these technologies in the hospital setting. This interest has been stimulated by four trends in the application of CGM technology, including (1) improvements in the technology and human factors of CGMs, (2) an increasing number of patients wearing these devices in ambulatory settings, (3) growing interest by clinicians to understand and interpret their hospitalized patients’ glucose concentrations, and (4) an accumulation of published reports describing use of these products in investigational settings. Diabetes Technology Society (DTS) previously organized guidance on the use of CGMs in the hospital as “Consensus Statement on Inpatient Use of Continuous Glucose Monitoring,”1 published in 2017. Because of recent increasing interest in this topic, coupled with advances in technology, DTS recognized a need for an updated consensus guideline on the use of CGMs and AID systems in an acute-care setting.
On April 23, 2020, DTS, led by Dr David Klonoff, convened the Continuous Glucose Monitor and Automated Insulin Dosing Systems in the Hospital: Consensus Guideline Panel. This international panel consisted of experts in diabetes technology from the United States, Europe, and Australia. The purpose of this meeting was to provide guidance for clinicians on how and when to best use both subcutaneous CGMs and AID systems, as well as to promote clinical research utilizing these devices.
The panel was planned in late 2019 before the first case of Coronavirus Disease 2019 (COVID-19) was reported. Two weeks prior to the panel meeting, two CGM companies announced that during the pandemic, the FDA had told them that the Agency would not object if these companies provided devices and technical support to hospitals who ordered CGMs for off-label use.2,3 Because some healthcare systems were interested in validating CGMs for use in their hospitals to preserve personal protective equipment (PPE) supplies and to minimize patient/provider contact, there was additional urgency for the panel to develop new clinical guidance. Panelists discussed how the pandemic has impacted inpatient glucose monitoring and how an urgent need has arisen for alternative approaches to this monitoring.4 The traditional approach of testing capillary BG every one to two hours in patients who are receiving intravenous insulin in an intensive care unit (ICU) as well as frequent BG testing in non-ICU wards for patients receiving subcutaneous insulin is not workable during the pandemic. Other methods are needed to decrease nurse contact with the patient for assisted monitoring of BG (AMBG)5 in order to (1) decrease risk of contagion from exposure to patients, (2) save time from donning and doffing PPE wherever possible, and (3) preserve limited supplies of PPE.4 Despite limited guidance, established studies, or widespread support from the clinical community to use CGMs in acute care,6 some healthcare professionals (HCPs) in the hospital diabetes community have recently begun to prescribe CGMs in the hospital setting for investigational or off-label use for COVID-19 patients.7
The Continuous Glucose Monitors and Automated Insulin Dosing Systems in the Hospital Consensus Guideline Panel included professionals from a variety of backgrounds. Members included experts in the use of CGMs from adult endocrinology, pediatric endocrinology, obstetrics and gynecology, advanced practice nursing, diabetes care and education, clinical chemistry, bioengineering, and product liability law. The expert panel included representatives from academia and government and observers from government (FDA), and industry (Abbott Diabetes Care, Dexcom, Glytec, Medtronic, and Roche Diagnostics). One member represented the College of American Pathologists, one represented the Endocrine Society, and one represented the Association of Diabetes Care and Education Specialists.
The expert panel discussed the following five topics: (1) continuation of home CGMs after hospitalization, (2) initiation of CGMs in the hospital, (3) continuation of AID systems in the hospital, (4) logistics and hands-on care of hospitalized patients using CGMs and AID systems, and (5) data management of CGMs and AID systems in the hospital (Table 1). Panelists reviewed available evidence on the inpatient use of diabetes technology, and discussed potential opportunities, potential barriers, and recommendations associated with the use of these devices in the hospital setting.
Table 1. The Five Topics Discussed at the Continuous Glucose Monitors and Automated Insulin Dosing Systems in the Hospital Panel.
| Topic 1: Continuation of home continuous glucose monitors after hospitalization Topic 2: Initiation of continuous glucose monitors in the hospital Topic 3: Continuation of automated insulin dosing systems in the hospital Topic 4: Logistics and hands-on care of hospitalized patients using continuous glucose monitors and automated insulin dosing systems Topic 5: Data management of continuous glucose monitors and automated insulin dosing systems in the hospital |
Recommendations were proposed by the panelists and then reviewed by the entire panel for favorability. Recommendations receiving at least 80% favorable votes were classified as strong recommendations, proposals receiving 60%-79% favorable votes were classified as mild recommendations, and proposals receiving less than 60% favorable votes were classified as recommendations that failed to receive consensus support.
For each of the five topics of this guideline (Table 1), six categories of recommendations (two for clinical practice, two for future research, and two for hospital policies) were developed for the main stakeholders of CGM and AID system technology in the hospital. These types of recommendations included (1) and (2) strong and mild recommendations that clinicians (HCPs or nursing) should do to utilize the technology optimally, (3) and (4) strong and mild recommendations that researchers and manufacturers need to do to improve the safety and effectiveness of the technology, and (5) and (6) strong and mild recommendations that hospitals need to do to build an environment for facilitating use of these devices. We define “should” as a statement of good practice and “need” as a necessary step to ensure patient safety or proper fulfillment of a procedure. These recommendations are intended to promote the best use of CGMs and AID systems in the hospital.
Background
CGMs were developed for the outpatient setting, and their transition for use in hospitals has been the subject of ongoing scholarship, research, and consensus guidelines. The first CGM became commercially available in 1999.8 CGM technology has greatly improved since then and several revolutionary developments in CGM technology have taken place over the past five years. These advances have all significantly reduced patients’ burden of diabetes care. The result has been improved patient satisfaction and self-care behaviors, increased clinician awareness, and a significant increase in CGM adoption, mostly by patients with type 1 diabetes mellitus (T1DM), but also in some patients with type 2 diabetes mellitus (T2DM).9 Software for analyzing continuous glucose data streams has permitted the development of new CGM-based glycemic metrics, which, compared to hemoglobin A1c, illustrate multidimensional patterns of glycemia more directly and with greater granularity.10 Improvements in CGM technology have also permitted integration with CSII systems to create AID systems. With the increasing popularity of AID systems that depend on CGMs, hospital HCPs will increasingly encounter patients who will want to utilize their CGMs and AID systems for inpatient diabetes care.
AID systems are becoming more advanced and are more frequently utilized for outpatients to successfully achieve glycemic outcomes in diabetes by facilitating increased time in range (TIR) and decreased time in hypo- and hyperglycemia. Two AID systems are currently cleared or approved by the FDA for home use in people with diabetes: 670G (Medtronic, Northridge, CA, USA) and Tandem Control IQ (Tandem Diabetes Care, Inc., San Diego, CA, USA). Some patients utilizing these AID systems and/or their physicians wish to continue the AID systems even during a hospitalization, believing that the benefits of commercial AID systems outweigh potential risks in this setting and noting that product use would not be off label if a patient is self-managing using the device even if the patient is in the hospital while doing it.
CGM sensors can be invasive (intravascular blood sampling or sensing devices that remove blood), minimally invasive (subcutaneous placement of a sensor), or noninvasive (transdermal CGMs that do not puncture the skin). They are measuring in different compartments, which can lead to different values.11 The frequency of receiving a signal by a CGM ranges from every 1 to 15 minutes, most commonly every 5 minutes. Invasive CGMs that are intended only for hospital use include two systems cleared by the FDA. They are (1) the GlucoScout (International Biomedical, Austin, TX, USA)12 and (2) the OptiScanner 5000 (OptiScan Biomedical Corporation, Hayward, CA, USA).13 Both devices track glycemic patterns of blood that is withdrawn from the venous system of adults.13 In Europe, four CGMs have been CE Marked for measuring venous blood in hospitalized patients: (1) GlucoClear (Edwards Life Sciences, Irvine, CA, USA),14 (2) Glysure System (Glysure, Abingdon, Oxfordshire, UK),15 (3) Eirus (Maquet Getinge Group, Rastatt, Germany),16 and (4) Optiscanner 5000.13 The Optiscanner 5000 has received FDA clearance, but the Glucoclear, Glysure System, and Eirus products all have not received FDA clearance. The Glucoclear and Eirus products have been discontinued, and Glysure Ltd. went out of business in 2018. The Optiscanner 5000 is available in the United States and Europe. One CGM with a subcutaneous sensor was available in Europe for measuring glucose in hospitalized patients: Sentrino Continuous Glucose Management System (Medtronic, Northridge, CA, USA).17 However, at this time, Sentrino is not a commercial product. There are no commercially available noninvasive CGMs in the United States.
In the hospital special issues can arise that can impair proper function of CGMs. No CGM is labeled to allow for exposure to X-rays, computed tomographic (CT) scans, magnetic resonance imaging (MRI), diathermy, radiation therapy, or other types of radiation. Typically, the device is removed or covered with a lead shield during these procedures. Some sites have covered their CGMs with a lead shield and have not reported adverse events. Emerging data suggest that there may be no need for removal of the Dexcom G6 sensor (Dexcom, San Diego, CA, USA) during X-rays, CT scans, radiation therapy, or when electrocautery is used during surgical procedures.18–20 There were no data errors observed when FreeStyle Libre Pro sensor was exposed to chest X-rays, CT, radiotherapy, and MRI.21 The panel expected that each manufacturer will continue to determine and report the impact of imaging studies and electrocautery on their particular devices.
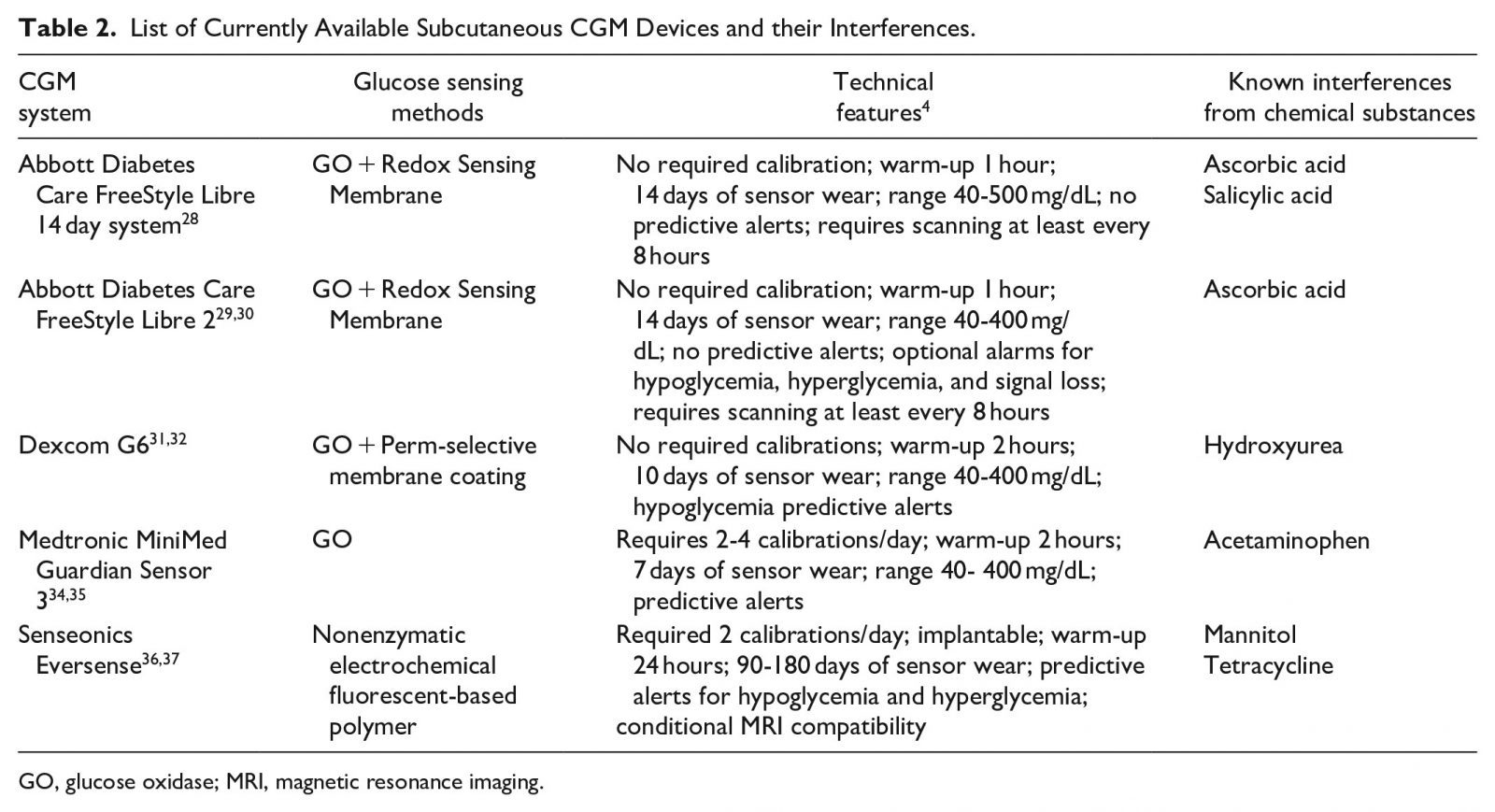
An attractive feature of CGMs is that they can measure glucose concentrations automatically and sound an alarm for readings that are outside of a prespecified safe target range. Five subcutaneous home-use CGMs are currently available with the potential for hospital use: FreeStyle Libre 14-day system,22 FreeStyle Libre 223 (both Abbott Diabetes Care, Chicago, IL, USA), Dexcom G6,24 Medtronic Guardian Sensor 325 (Medtronic Diabetes, Northridge, CA, USA), and Eversense (Senseonics, Inc., Germantown, MD, USA).26 Table 2 presents these devices’ glucose sensing methods, technical features, and known interferences from chemical substances.27–37
Continuation of Home CGMs After Hospitalization
Chair: Robert J. Rushakoff, MD
University of California, San Francisco, CA, USA
Potential Opportunities
Patient Considerations
Standalone CGMs and AID systems are typically used in the outpatient setting. If a patient wearing either of these technologies is hospitalized, then policies are needed to continue these technologies. Some hospitals have policies for removing personal use devices like CGMs, CSII systems, and AID systems from patients when they are admitted. It is within the FDA’s authorized use for a patient to use their own device for self-management while in a hospital. What is not authorized is when a hospital wants to use the CGM for their own testing purposes as well as in patients who do not have diabetes.
This section focuses on continuing a CGM already started before a patient arrives at the hospital and a subsequent section focuses on initiating a CGM in the hospital. Anyone with diabetes who is using a CGM and who is not cognitively impaired is a candidate to continue with this device in the hospital
Benefits of CGMs
Several studies have demonstrated that CGMs in ambulatory settings improve patients’ satisfaction,38,39 as well as control (eg, better TIR and time in hypo- and hyperglycemia).40,41 Continuation of an outpatient CGM during a hospitalization could improve patient satisfaction and efficacy of glycemic monitoring by assisting the patient and the hospital staff to identify glucose patterns, and predict future glycemia with trend arrows and rate of change,42 and potentially prevent severe hypo- and hyperglycemic events.43 This would be particularly relevant if staffing shortages exist or a patient is no longer aware of hypoglycemia. Accordingly, asking patients to remove their CGMs in the hospital could potentially contribute to decreased patient satisfaction and quality of care. CGM use in ICU and non-ICU settings has several superior features over intermittent point-of-care (POC) testing for glucose monitoring during continuous insulin infusion and subcutaneous insulin therapy, and possibly is a safer and less costly approach that can reduce workload. Additionally, CGM technology could potentially replace many uses of POC capillary BG testing in the hospital.43 However, if CGM readings turn out to be inaccurate, then more confirmatory testing would be needed and that could increase workload.
Pregnancy
The use of CGMs in pregnant patients with T1DM has been associated with improvement in both maternal and fetal outcomes in five areas, including (1) time in glycemic target range without increase in hypoglycemia, (2) lower incidence of large-for-gestational-age babies, (3) fewer neonatal ICU admissions, (4) reduced neonatal hypoglycemia, and (5) decreased length of stay (LOS).44,45 The use of CGMs in pregnancy is considered off-label in the United States, but not in Europe. In recent years, patients and HCPs have identified real-time continuous glucose monitoring as a helpful adjunct. Although there is ongoing interest in the use of CGMs in pregnancy, there are limited data about its use in the acute-care setting. If an HCP intends to use such a device, then it would be important to avoid placing it near areas of potential obstetric surgery.
Potential Barriers
Studies on substances that interfere with current subcutaneous CGMs are shown in Table 2. The panel agreed that CGM results should be interpreted cautiously in patients using select drugs known to cause interference with CGM sensing technologies. For these situations, panelists recommended using more accurate glucose testing, such as laboratory analyzers or AMBG5 using hospital BG monitors (BGMs; which, unlike home-use BGMs, require special cleaning and disinfection procedures). Even though these devices are factory-calibrated and a limited set of studies have reported acceptable accuracy in critically ill patients,46 several potential scenarios in the hospital (eg, interfering substances, hypoxia, acidosis, and hypotension) would require very careful use of this technology. The panel did not feel that current CGMs can now replace capillary POC finger stick monitoring or other FDA-cleared methods for monitoring BG in the hospital.
Recommendations for Continuation of Home CGMs After Hospitalization
Clinical Practice
Strong Recommendations
- HCPs should consult with an inpatient diabetes team if available, when continuing or initiating a CGM or AID system.
- HCPs should avoid relying on CGM data for glycemic management decisions in patients with severe hypoglycemia or hyperglycemia (ie, BG < 40 mg/dL or > 500 mg/dL).
- HCPs should avoid using CGMs for management of (1) diabetic ketoacidosis until glucose is in the CGM measurement range, and then CGMs should be used adjunctively or (2) situations with rapidly changing glucose levels and fluid/electrolyte shifts.
- HCPs should avoid continuing or initiating CGMs to patients with skin infections near the sensor site or placing sensors in areas with significant edema as well as patients treated with vasoactive agents or poor tissue perfusion.
- HCPs should use a CGM checklist for elective procedures during the preoperative visits to ensure proper documentation of devices and real-time data reporting.
- HCPs should advise pregnant women to continue the use of a CGM during a hospitalization to identify glucose trends and prevent hypo- or hyperglycemia.
- HCPs should instruct patients to bring supplies with them to the hospital for the duration of any preplanned admission or elective procedures.
- HCPs should check capillary BG or serum BG concentrations after procedures for non-critically ill patients and venous/arterial blood for critically ill patients to ensure the patient’s CGM is functioning properly.
- HCPs should use trend arrows and rate of change to help prevent extreme glycemic excursions and (when a CGM is used adjunctively) to help determine when a BG test is required.
- HCPs should set alarm thresholds for inpatient glycemic targets, such as predicting hypoglycemia (typically BG < 80-85 mg/dL) or predicting hyperglycemia.
- Nursing should document CGM and/or CSII system information in the electronic health record (EHR) for all admissions or elective procedures.
Research
Strong Recommendations
- Researchers need to provide more data to support definitive recommendations on improved outcomes for continuation of home/ambulatory CGM use after hospitalization.
- Researchers need to conduct studies on the roles of CGM and POC BG testing and identify the optimal features of telemetry to inform nursing staff about actionable CGM patterns.
- Researchers need to perform further studies to assess the accuracy of CGMs during pregnancy, labor and delivery, and the peripartum period.
- Researchers need to study the impact of lag time on glucose measurements (ie, situations with rapid changes in the glucose concentration) in the hospital.
Hospital Policies
Strong Recommendations
- Hospitals need to develop standard CGM data reports and workflows.
- Hospitals need to implement policies for testing capillary BGs and calibrating CGMs if the CGM requires calibration.
- Hospitals need to develop a system for automatic staff notification for CGM alarms that predict impending or current hypoglycemia or hyperglycemia.
- Hospitals need to develop specific guidelines for using CGMs and AID systems for their affiliated nursing homes and skilled nursing facilities.
Initiation of CGMs in the Hospital
Chair: Guillermo E. Umpierrez, MD, CDE
Emory University School of Medicine, Atlanta, GA, USA
Potential Opportunities
COVID-19
The current COVID-19 pandemic created the need for innovative approaches for glycemic monitoring in the hospital.4 Coincidentally, two weeks before this meeting, the FDA stated that they would exercise enforcement discretion and they would not object to the use of CGMs in the hospital during the crisis.2,3 This policy was intended for the factory-calibrated CGMs manufactured by Abbott Diabetes Care and Dexcom. Subsequently, these two manufacturers provided CGM supplies to hospitals to help monitor glucose remotely. Immediately afterward, several institutions started the process of implementing CGM use and realized that there was a need for training, implementation, and resource utilization and not all hospitals have this expertise. The announcement also resulted in new reports on the use of CGMs in the hospital. During the panel discussion, there was a recognition that this “exceptional” situation did not indicate “label approval” for CGM use in the hospital by regulatory bodies. Collaborative efforts from Emory University and DTS have recently provided examples of practical implementation of CGMs and use of diabetes technology in the hospital through creation of a website that contains information about original articles, commentary, news, and protocols related to COVID-19 and diabetes47 (covidindiabete.org). Small pilot studies have provided unconfirmed evidence of the feasibility of remote glucose monitoring during this global crisis.40
ICU Patients
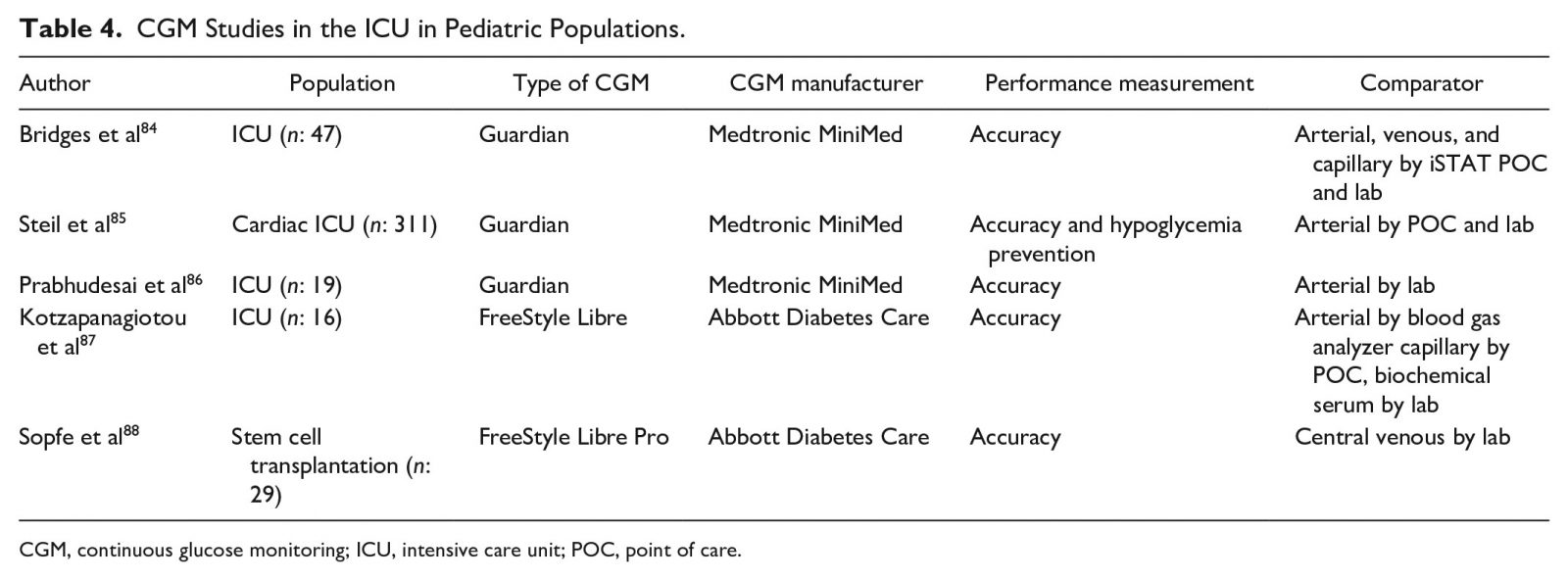
There is strong evidence from large, prospective, and randomized studies indicating that optimal glucose management results in improved outcomes, reduced complications, and a decreased LOS.48,49 In the ICU setting, therapy with intravenous insulin infusion allows clinicians to maintain narrow glycemic targets. The panelists reviewed studies using CGMs in the ICU in adult populations (Table 3) 50–83 and pediatric populations (Table 4) 84–88.
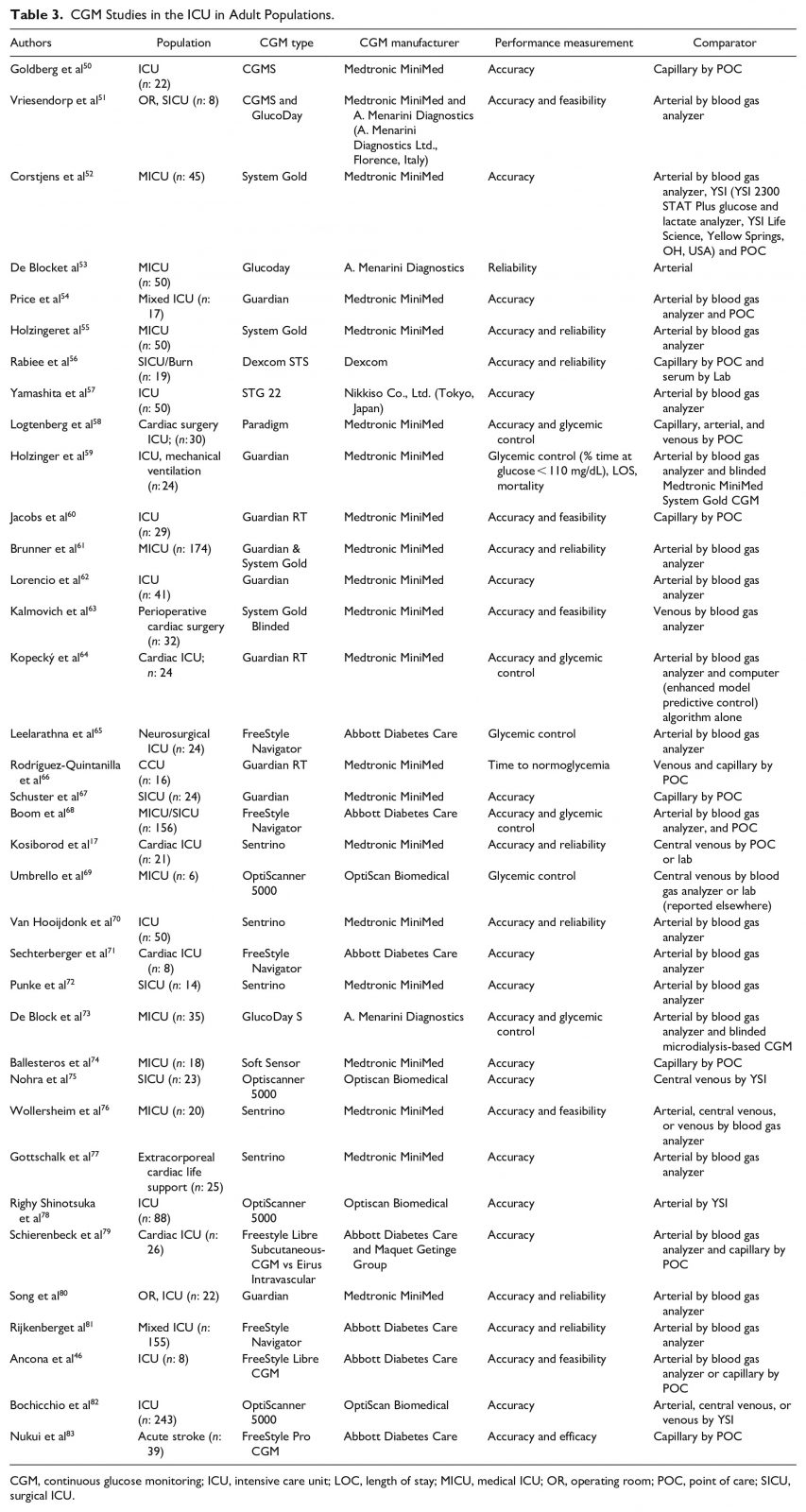
In the ICU, bedside POC glucose using factory-calibrated BGMs (performed every one to two hours) has been recommended as the preferred method to assess glycemic management and to guide hyperglycemia treatment with intravenous insulin infusion. POC BG testing has drawbacks. This testing method is labor-intensive. Also, POC testing does not provide (1) a full 24-hour glycemic profile, (2) predictions of hypoglycemic events, or (3) alarms for asymptomatic hypo- or hyperglycemia. Although the use of POC glucose testing, compared to central laboratory glucose testing, is approximately as convenient and generates faster results, another drawback is that it costs more. Estimated mean total costs (including equipment, supplies, and labor) can be up to $5.13 per POC test in a high-test-volume nursing unit, and up to $16.49 per POC test in a low-test-volume nursing unit, compared to $3.78 for central laboratory glucose testing.89 Moreover, the accuracy of POC glucose meters is not optimal, with only 6 of 18 glucose monitor systems (representing 90% of commercially available meters and intended for outpatient use) meeting regulatory accuracy requirements17 in a recent study. In 2018, the FDA cleared the first POC glucose meter—the StatStrip Glucose (Nova Biomedical, Waltham, MA, USA)—for all hospitalized patients, including critically ill patients, to test capillary, venous, and arterial blood specimens.90 However, not all hospitals use this system to measure BG. While definitive validation of CGM accuracy in ICU patients is still forthcoming, there remains a potential role for CGMs to measure glucose concentrations in this population.46,91,92
Non-ICU Patients
Studies using older CGM technology that required regular recalibration have shown minimal differences in mean daily glucose, premeal, fasting, or two-hour postprandial glucose levels between CGM and POC BG testing. In a pilot study, CGMs detected a higher number of hypoglycemic events compared to POC BG testing, particularly nocturnal or asymptomatic hypoglycemia.93 Few studies have been published on the use of newer factory-calibrated CGMs in non-ICU settings.94
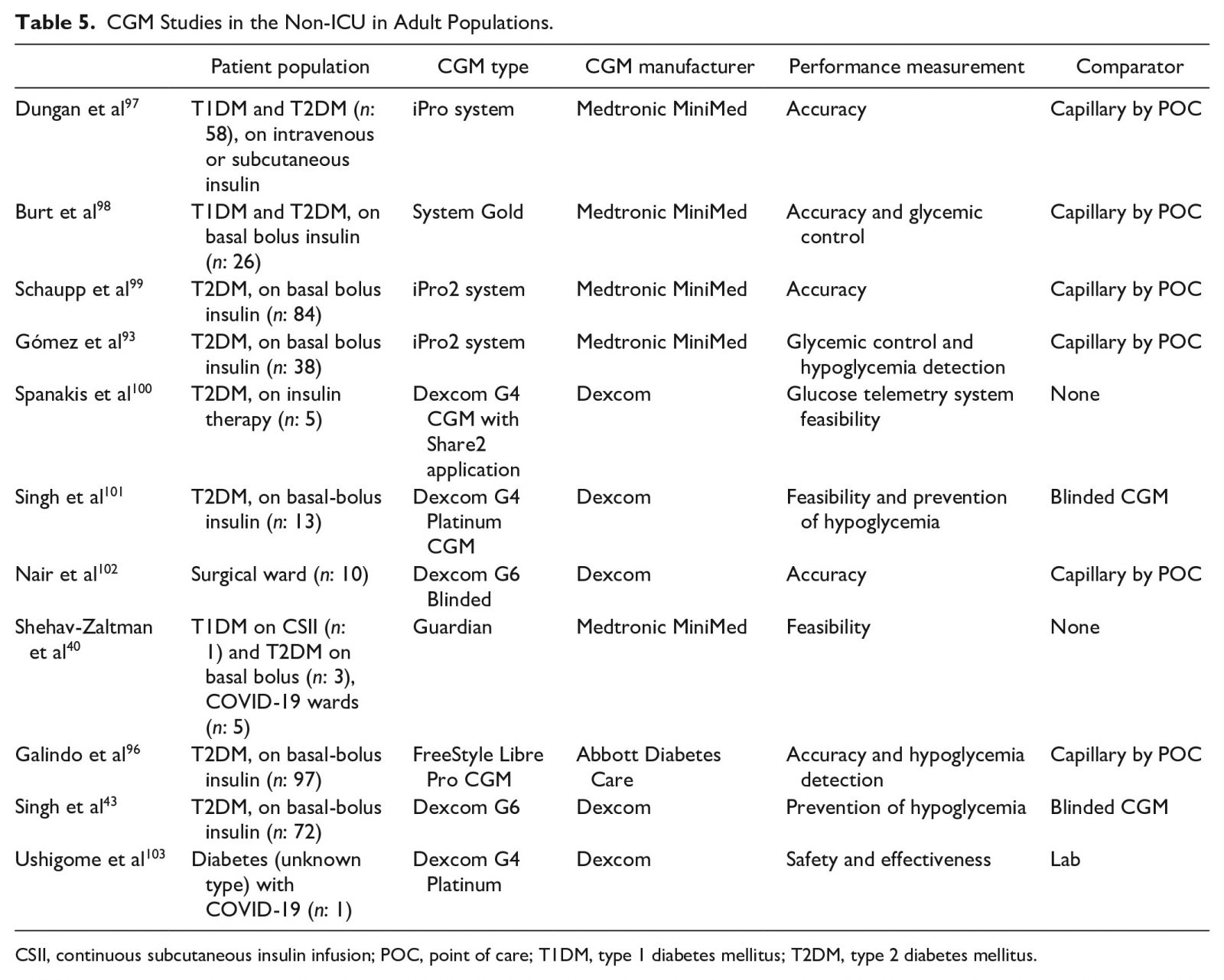
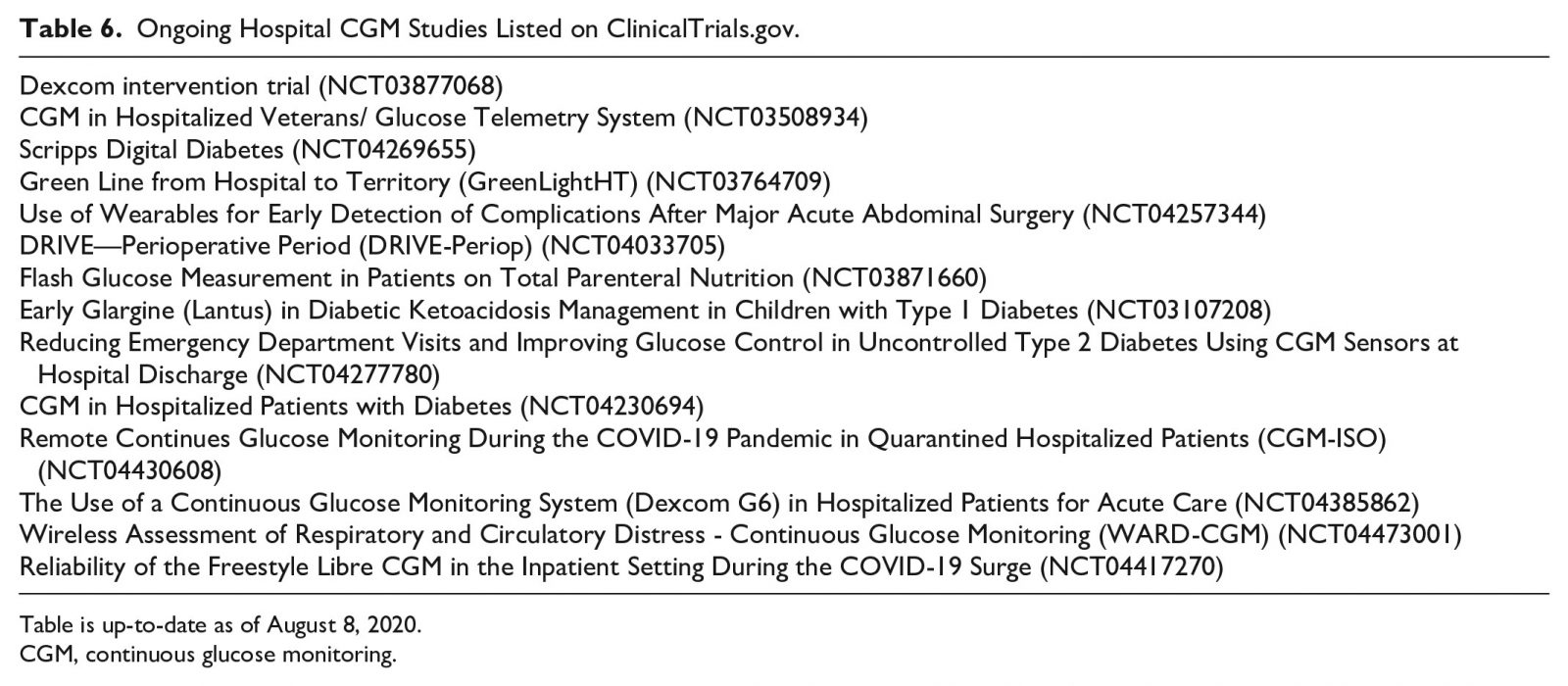
A recent study of patients with T2DM admitted to general medicine and surgery wards and managed with basal-bolus insulin therapy compared the FreeStyle Libre Pro (Abbott Diabetes Care, Alameda, CA, USA)95 to POC BG testing.96 This CGM system is a variant of the FreeStyle Libre 14-day system, where glucose readings are available to the HCP but not to the patient. The FreeStyle Libre Pro CGM, compared to POC BG testing, showed a tendency toward lower mean glucose with an estimated mean glucose difference of 12.8 mg/dL (confidence interval [CI] 8.3-17.2). Accordingly, CGMs, compared to POC BG testing, were more sensitive at detecting hypoglycemic events. The overall mean absolute relative difference was 14.8%. The percentage of glucose concentrations within the ±15% or 15 mg/dL, ±20% or 20 mg/dL, and ±30% or 30 mg/dL (where for CGM concentrations ≤100 mg/dL, the units of the range were mg/dL and for CGM concentrations >100 mg/dL, the units of the range were percent) was 62%, 76%, and 91%, respectively. A Clarke error grid analysis showed acceptable clinical accuracy with 98.0% of glucose concentrations falling into Zones A (75.1%, n = 1184) and B (23.7%, n = 374).96 Panelists reviewed CGM studies in the non-ICU in adult populations (Table 5). 40, 43, 93, 96–103 Evidence suggests that initiating the use of CGMs in the non-ICU settings provides better glycemic monitoring, compared to standard 3-4 times daily POC BG testing, with improved detection and potential prevention of hypo- and hyperglycemic events. Most of these events, particularly nocturnal and asymptomatic hypoglycemia, might otherwise be missed. Ongoing hospital CGM studies listed on ClinicalTrials.gov104 may provide some guidance (Table 6).
Glucose Telemetry
The hospital should possess the physical infrastructure to download the patient’s CGM data for the retrospective review of patterns in glycemia. CGM data can be automatically delivered to the nursing station by way of automatic downloading into a monitor at the nursing station. A recently published manuscript evaluated whether such a system for presenting CGM data, called the “Glucose Telemetry System,” can decrease hypoglycemia in the general wards/non-ICU setting.43 This report is the first interventional randomized controlled trial (RCT) study of CGM technology to improve outcomes in the non-ICU setting. The study included patients with T2DM, who were at high risk for hypoglycemia. Participants were randomized to either the “Glucose Telemetry System” (intervention group) or to POC BG testing (control group). For patients in the “Glucose Telemetry System,” nurses were instructed to proceed with hypoglycemia prevention actions if the low-glucose alerts were activated (for a setting of BG < 85 mg/dL). Participants in the control group were placed on “blinded” CGMs, which were only used to collect glucometric data. Overall, the subjects in the “Glucose Telemetry System” experienced fewer events of hypoglycemia (BG < 70 mg/dL) and clinically significant hypoglycemia (BG < 54 mg/dL) compared to the POC BG group. The outcomes of the intervention versus control groups for these two levels of hypoglycemia were, respectively, 0.67 versus 1.69 events/patient, P = .024 (BG < 70 mg/dL) and 0.08 versus 0.75 events/patient, P = .003 (BG < 54 mg/dL). There was a reduction in percentage of time in hypoglycemic range (BG < 70 mg/dL and <54 mg/dL) in the glucose telemetry system group compared to POC group (0.40% vs 1.88%, P = .002 and 0.05% vs 0.82%, P = .017).
Potential Barriers
Minimally Invasive CGMs
As discussed in previous consensus reports1,105 during the past 20 years, many studies have been published on the initiation of subcutaneous CGMs in critically ill patients (Tables 3 and 4). However, most of those studies were intended to focus only on accuracy data and not clinical outcomes. In addition, it is difficult to reach conclusions from these reports because of different study designs and small sample sizes. A recent systematic review by van Steen et al analyzed 32 studies that assessed the accuracy of CGMs in the ICU. These authors reported moderate-to-good accuracy especially with intravascular devices.106 The authors included only five RCTs for efficacy assessment and recognized methodological limitations.106 Panelists noted that there is currently insufficient data to provide definitive recommendations on improved outcomes based on reports in the medical literature.
It is unclear whether CGMs will be able to fully replace POC BG testing and be approved as nonadjunctive use for treatment decisions in acute care. Panelists had concerns with the accuracy of subcutaneous CGM values for the first hours after insertion to make treatment decisions or even during the first one to two days of use. Panelists also had concerns with the unintentional added burden on nursing when (1) a CGM has overreported low glucoses values and these false low values have required POC confirmation, (2) new CGM technology must be learned during a crisis, and (3) time is needed for troubleshooting. In addition, skin-related issues have been mentioned in 19% of articles about recent CGMs.107–109
Invasive CGMs
Although these systems were not the focus of the guideline, the panelists briefly considered the role of invasive CGMs. They noted that few intravascular invasive sensors are cleared for ICU patients. Also, compared to subcutaneous CGM sensors, intravascular sensors tend to have three main disadvantages. First, these systems are invasive and some are associated with vascular complications, such as thrombosis, catheter occlusion, biofilm formation, or intravascular catheter-related infections.110 Second, they impose a higher implementation resource and care burden to patients and the ICU system. Third, they are not intended for non-ICU settings. Therefore, intravascular CGMs, compared to subcutaneous CGMs, are less attractive options.
Recommendations for Initiation of CGMs in the Hospital
Clinical Practice
Strong Recommendation
- HCPs should consider prescribing CGMs to reduce the need for frequent nurse contact for POC glucose testing and the use of PPE for patients on isolation with highly contagious infectious diseases (eg, COVID-19).
Mild Recommendation
- HCPs should avoid initiating CGMs in patients with severe hypoglycemia or hyperglycemia (ie, BG < 40 mg/dL or > 500 mg/dL) or during periods of rapid glucose fluctuations.
Research
Strong Recommendations
- Researchers need to provide data to support initiation of CGMs for improving patient-centered outcomes.
- Researchers need to provide data on hospital outcomes when initiating CGMs in the hospital, including improved glycemic outcomes, detection and/or reduction of hypoglycemia and hyperglycemia, reduction of ICU LOS, and cost-effectiveness.
- Researchers need to conduct studies on long-term benefits for initiating CGMs in the hospital after discharging patients with newly diagnosed diabetes or recurrence of diabetic ketoacidosis (DKA) or other complications of diabetes.
- Manufacturers need to develop educational tools for patients, hospital staff, and HCPs.
Hospital Policies
Strong Recommendations
- Hospitals need to develop plans, including process maps, protocols, staff educational resources, and order sets for prescribing CGM use during hospitalizations before implementing a CGM.
- Hospitals need to provide educational tools for patients, nurses, house staff, and attending physicians when a patient in the hospital starts on a CGM.
Continuation of AID Systems in the Hospital
Chair: Ananda Basu, MD, FRCP
University of Virginia School of Medicine, Charlottesville, VA, USA
Potential Opportunities
Improved Glycemic Outcomes
Evidence about the potential glycemic benefits of continuing AID systems from the outpatient into the inpatient setting is limited, and currently it is possible only to extrapolate data from studies of AID systems initiated during a hospital stay. Several such studies of initiating AID systems in the hospital have been performed in medical or surgical patients as well as in patients on hemodialysis or women in the peripartum/postpartum period.111–118 In the largest of these studies,111 Bally et al reported that initiation of AID system technology in the hospital for patients receiving noncritical care achieved a higher percentage of TIR when compared to standard hospital management. The times in range were, respectively, 65.8 (±standard deviation 16.8)% vs 41.5 (±16.9)%, with a difference of 24.3 (±2.9)% (95% CI 18.6-30.0; P < .001). Mean glucose levels were lower in the AID system arm compared to the group treated with conventional subcutaneous insulin delivery (with the differences being 154 [±29] mg/dL vs 188 [±43 mg/dL], P < .001) and there was no significant difference in time spent in hypoglycemia, <54 mg/dL or <70 mg/dL. AID systems have also been found to improve TIR in women in the peripartum/postpartum period112 and patients on hemodialysis.113 AID system management has reduced surgical site infections resulting in shorter postoperative hospitalizations.114 In a single-center observational study that was performed in an ICU setting, use of AID system management compared to standard sliding-scale insulin therapy led to a decreased frequency of blood sampling, reduced time required for achieving glycemic targets, and a decreased nursing workload per admission of diabetes management from 68 (±25) minutes (AID system) to 33 (±21) minutes (sliding scale) (P < .001).115 In a randomized, parallel-group trial, inpatients with T2DM in the United Kingdom received fully closed-loop insulin delivery without meal-time boluses, which was found to be safe and effective.116 In a two-center open-label, RCT of fully AID in the United Kingdom and Switzerland, this method was found to improve glycemic outcomes for inpatients receiving nutritional support.117
Glycemic management in hospitalized patients aims to avoid both hypoglycemia and hyperglycemia. Since patients with diabetes are often in a compromised state of health and at risk for hypoglycemia because of interrupted nutrition, inadvertent insulin overdosages associated with intensive insulin therapy, or unexpected improvements in insulin sensitivity, hypoglycemia can be a serious problem for these patients. Special AID systems that can deliver both insulin and glucose have been created exclusively for inpatient use. A clinical study in Japan compared two such systems (differing in size and weight, but not algorithms) manufactured by Nikkiso Co., Ltd., and used for perioperative glycemic management. The newer (STG-55) and older (STG-22) AID system models119 both achieved similar glycemic control without hypoglycemia, leading the investigators to conclude that the newer (as well as smaller and lighter) system could potentially be used in routine practice for perioperative glycemic management.118 A study in Denmark assessed an intravenous AID infusion system delivering both insulin and glucose based on a proprietary controller (Admetsys, Boston, MA, USA).120
COVID-19
With the COVID-19 pandemic, increased mortality has been associated with hyperglycemia both in patients diagnosed with diabetes prior to admission and those diagnosed with diabetes during their admission.121 There is a paucity of high-quality data about optimal monitoring and therapy and associated outcomes in these patients. The need for improved glycemic management for COVID-19 patients may accelerate the development of future novel glucose monitoring technologies in the hospital setting, including possibly closed-loop control for intensively treated patients. During the pandemic, AID systems, if utilized, can also perhaps reduce the risk of nursing exposure, the time needed for donning and doffing for any needed BG monitoring, and the use of limited supplies of personal protective equipment.
Patient Satisfaction
Evidence about the potential benefits of using of AID systems in the inpatient setting is limited. Even for the more traditional non-AID CSII system, the available data are based on retrospective studies, because no randomized clinical trials have been performed.122 One of these studies reported that outpatients on CSII systems, who had reasonable control (mean hemoglobin A1c 7.5%),123,124 were sufficiently confident to continue self-managing their diabetes and use their own CSII systems during a hospitalization. Many of these CSII system users reported higher patient satisfaction (86%) when they were allowed to continue wearing their CSII system during their inpatient stay.125 Similar outcomes are likely to be found with the use of AID systems. Asking hospitalized patients with diabetes to remove their AID system could result in decreased patient satisfaction, especially if their diabetes care is managed by healthcare professionals, who have limited experience with inpatient and outpatient diabetes management. Furthermore, a patient who must surrender their AID system upon hospitalization might express dissatisfaction with nocturnal POC BG testing.
Potential Barriers
Patient-Related Factors
Although AID systems can be beneficial, five types of factors may preclude their use in the inpatient setting.122,123,126 They can be divided into the following categories: (1) patient-related, (2) hospital-related, (3) device-related, (4) medication-related, and (5) surgical procedure-related. Examples of patient-related conditions in which AID systems should not be used are physical or psychiatric conditions, which can make patients incapable of self-managing an AID system in the hospital. Contraindications to CSII system and AID system therapy in the hospital are presented in Table 7. Patients should be able to self-manage their AID systems and provide their pump settings to the treating HCPs in case the AID system may need to be discontinued. Patients with severe metabolic decompensations, such as DKA,122 acute kidney injury, post-transplant T1DM patients in acute rejection, or those with severe sepsis and hypovolemia, which may lead to tissue hypoperfusion, should also probably not use AID systems in the hospital. Skin infections may represent another contraindication, especially if they are extensive, because they may preclude CGM or pump placement. However, it is still unclear whether the above conditions can significantly affect the function of AID systems and more research is needed in this area.
Hospital-Related Factors
Examples of hospital-related factors are situations where there are no policies in place that can safeguard the use of AID systems in the inpatient setting and delineate the roles of the patients, nurses, and HCPs.123,126 Because only limited information is currently available about the use of AID systems in the hospital, further research is needed in order to provide evidence-based recommendations.126 Another potential obstacle to the use of AID systems in the inpatient setting is the lack of nurses and HCPs who are adequately trained in the use and interpretation of data from the AID systems. However, it is unclear whether AID systems do or do not lead to increased workload for nursing and/or HCPs.
Device-Related Factors
Limitations related to device use include clinical scenarios where AID systems cannot be used because of a device malfunction or insufficient medical supplies, either for the continuous insulin infusion set or for the CGM components. A CGM can become compressed during a prolonged period of a prone position, such as with sleep or prone ventilation, and produce a false low reading, which could also pose another limitation to their use.127,128 For AID systems that require the patient to select a meal-time bolus dose recommended by a bolus calculator, unexpected failure to reach postprandial glycemic targets could be due to manufacturer-specific pump settings resulting in a different dose recommendation by each pump brand.129
Medication-Related and Meal-Related Factors
Medications, such as glucocorticoids, which can cause severe insulin resistance and uncontrolled hyperglycemia, may present a challenge for some AID systems, but others may adapt well to changes in insulin resistance during periods of illness.130 Other challenging scenarios are nutritional interruptions, which are very common in a busy hospital environment.130 Nutrition in the inpatient setting is more complicated than in the ambulatory environment. Patients may have nausea, vomiting, or other conditions that can affect nutrient absorption and therefore create irregular patterns in the glucose values. Insulin is not always administered at the right time before the meal is delivered. Meals can be interrupted or delayed and tube feedings and parenteral nutrition (either peripheral or total) can be suddenly discontinued. Although the above scenarios are not absolute treatment-related contraindications, they represent challenging situations for AID system use in the hospital. HCPs should also be aware about the potential interactions of certain medications with subcutaneous CGMs (Table 2). Additional studies are required to determine the effects, if any, of multiple doses and combinations of potentially interfering medications on CGM accuracy.
Surgical Procedure-Related Factors
Surgical procedures can create additional barriers to the use of AID systems in the inpatient setting.122,124,131 Surgical procedures can be broadly divided into two different categories, elective or urgent. Elective surgeries can provide sufficient time for preadmission preparation. The endocrinology clinician or diabetes team would coordinate care between the different subspecialties that are involved such as the anesthesiology, and surgical and inpatient diabetes teams (if they are available and different from the primary endocrinologist) about the upcoming surgical procedure. The panel recognized that many hospitals do not have a diabetes team or inpatient diabetes educator. Patients need to be instructed to insert the sensor and the insulin cannula away from the operative field and change the sites one day prior to the surgery. Urgent surgeries do not allow for such planning. In the immediate preoperative period, for either elective or urgent surgical procedures, the inpatient diabetes team should be notified, if this has not been done earlier. Consent must be obtained from the patient about the use of an AID system during surgery. Temporary higher glycemic targets may be needed to allow slightly higher glucose values during surgery to decrease the risk of hypoglycemia in an unconscious patient. Ideally, the anesthesiology team would need to be familiar with the use of an AID system during the intraoperative period so the team can control or suspend the pump if necessary because the unconscious patient will not be able to adjust the settings themselves. However, it is unclear whether it would be realistic to expect an anesthesiologist to learn the operation of an AID system and there is no data about anesthesiologists operating AID systems during surgery. The basal insulin delivery rate is determined by an AID system controller. If the team members are able to manage the AID system, then they should also have easy access and proximity to the AID system intraoperatively. The use of an AID system during surgery is not recommended if the insulin requirements are expected to fluctuate significantly intraoperatively. In that case intravenous insulin delivery with insulin dosing software instead of subcutaneous insulin delivery would be more appropriate with either an intravenous or subcutaneous glucose sensor. AID systems can be continued during the operation if there are no concerns regarding device malfunction. However, there are no good data available on the safety or maximum safe duration of closed-loop control during anesthesia. Even with control by an AID system, BG concentrations should be monitored intraoperatively.
Recommendations for Continuation of AID Systems in the Hospital
Clinical Practice
Strong Recommendations
- HCPs should prescribe AID systems only for appropriate candidates, who will need to have adequate knowledge and skills for using AID systems.
- HCPs should reassess a decision periodically to transition use of outpatient AID systems into the hospital in order to ensure that AID system continues to represent the best treatment option for each patient.
- HCPs should prepare an alternative plan for diabetes management in case it becomes inappropriate for a patient to continue using an AID system in the hospital.
- HCPs should discontinue AID systems in critically ill hospitalized patients (such as those with hypovolemia or sepsis).
- HCPs should recognize glycemic patterns due to CGM compression, which can cause false low readings.
Mild Recommendation
- HCPs should avoid initiating an AID system during a hospitalization.
Research
Strong Recommendations
- Researchers need to conduct studies about whether continuing AID systems in the hospital is beneficial to improve glycemic management or clinical outcomes.
- Researchers need to provide data on hospital outcomes when using AID systems in the hospital, including improved glycemic outcomes, detection and/or reduction of hypoglycemia, reduction of ICU LOS, and cost-effectiveness.
- Manufacturers need to research whether all types of CGMs and AID systems can be used during radiological/imaging studies or diathermy.
Hospital Policies
Strong Recommendations
- Hospitals need to develop institution-specific protocols and order sets for the proper use of AID systems during a hospitalization.
- Hospitals need to require that patients using AID systems bring with them sufficient supplies for these devices during a hospitalization.
- Hospitals need to develop protocols for using AID systems during elective procedures and surgeries.
Recommendation Not Reaching Consensus
- HCPs should switch AID systems from “auto” mode to “manual” mode when a patient is admitted to the hospital wearing an AID system.
Logistics and Hands-On Care of Hospitalized Patients Using CGMs and AID Systems
Chair: Suzanne Lohnes, MA, RN, CDCES, CPT
University of California San Diego Medical Center, La Jolla, CA, USA
Potential Opportunities
Expectations for Patients and Hospital Staff and Practical Considerations for Use of CGMs and AID Systems in the Acute Care Setting
Continuation of CGM use can be a helpful adjunct to management in the acute care setting and can increase patient satisfaction. However, because CGMs are not currently cleared by FDA for the inpatient environment, a policy addressing practical considerations for use of CGMs and AID systems in hospitalized patients is needed.
Potential Barriers
Necessary Hospital Responsibilities
It is important that key tasks, roles, and responsibilities, related to work system domains (technology/data, tasks, personnel, structure/organization, and environment), are addressed for safe and effective implementation.132 Below are listed potential responsibilities delineated by team members. It is helpful for diabetes team members to be interchangeable (eg, subspecialty consultant with pharmacist or nurse with patient care technician). Furthermore, it is appropriate to predefine tasks, person assignments, policies, procedures, and a clear organizational structure (eg, determination of committee reporting) around monitoring and interpretation of data, to facilitate use of CGMs and AID systems.
Necessary Patient Responsibilities
Patients who wish to continue use of CGMs or AID systems in the acute care setting should read a detailed set of information and should review and sign a patient agreement about hospital policy. The panel developed a sample patient agreement for the use of CGMs or AID systems in the hospital presented in Figure 1. This agreement is meant to be an example for a subcutaneous non-implanted sensor. Each institution must develop their own agreement and they should consider manufacturer labeling.

Hospital-Related Factors
Examples of hospital-related factors are situations where there are no policies in place that can safeguard the use of AID systems in the inpatient setting and delineate the roles of the patients, nurses, and HCPs.123,126 Because only limited information is currently available about the use of AID systems in the hospital, further research is needed in order to provide evidence-based recommendations.126 Another potential obstacle to the use of AID systems in the inpatient setting is the lack of nurses and HCPs who are adequately trained in the use and interpretation of data from the AID systems. However, it is unclear whether AID systems do or do not lead to increased workload for nursing and/or HCPs.
Device-Related Factors
Limitations related to device use include clinical scenarios where AID systems cannot be used because of a device malfunction or insufficient medical supplies, either for the continuous insulin infusion set or for the CGM components. A CGM can become compressed during a prolonged period of a prone position, such as with sleep or prone ventilation, and produce a false low reading, which could also pose another limitation to their use.127,128 For AID systems that require the patient to select a meal-time bolus dose recommended by a bolus calculator, unexpected failure to reach postprandial glycemic targets could be due to manufacturer-specific pump settings resulting in a different dose recommendation by each pump brand.129
Medication-Related and Meal-Related Factors
Medications, such as glucocorticoids, which can cause severe insulin resistance and uncontrolled hyperglycemia, may present a challenge for some AID systems, but others may adapt well to changes in insulin resistance during periods of illness.130 Other challenging scenarios are nutritional interruptions, which are very common in a busy hospital environment.130 Nutrition in the inpatient setting is more complicated than in the ambulatory environment. Patients may have nausea, vomiting, or other conditions that can affect nutrient absorption and therefore create irregular patterns in the glucose values. Insulin is not always administered at the right time before the meal is delivered. Meals can be interrupted or delayed and tube feedings and parenteral nutrition (either peripheral or total) can be suddenly discontinued. Although the above scenarios are not absolute treatment-related contraindications, they represent challenging situations for AID system use in the hospital. HCPs should also be aware about the potential interactions of certain medications with subcutaneous CGMs (Table 2). Additional studies are required to determine the effects, if any, of multiple doses and combinations of potentially interfering medications on CGM accuracy.
Surgical Procedure-Related Factors
Surgical procedures can create additional barriers to the use of AID systems in the inpatient setting.122,124,131 Surgical procedures can be broadly divided into two different categories, elective or urgent. Elective surgeries can provide sufficient time for preadmission preparation. The endocrinology clinician or diabetes team would coordinate care between the different subspecialties that are involved such as the anesthesiology, and surgical and inpatient diabetes teams (if they are available and different from the primary endocrinologist) about the upcoming surgical procedure. The panel recognized that many hospitals do not have a diabetes team or inpatient diabetes educator. Patients need to be instructed to insert the sensor and the insulin cannula away from the operative field and change the sites one day prior to the surgery. Urgent surgeries do not allow for such planning. In the immediate preoperative period, for either elective or urgent surgical procedures, the inpatient diabetes team should be notified, if this has not been done earlier. Consent must be obtained from the patient about the use of an AID system during surgery. Temporary higher glycemic targets may be needed to allow slightly higher glucose values during surgery to decrease the risk of hypoglycemia in an unconscious patient. Ideally, the anesthesiology team would need to be familiar with the use of an AID system during the intraoperative period so the team can control or suspend the pump if necessary because the unconscious patient will not be able to adjust the settings themselves. However, it is unclear whether it would be realistic to expect an anesthesiologist to learn the operation of an AID system and there is no data about anesthesiologists operating AID systems during surgery. The basal insulin delivery rate is determined by an AID system controller. If the team members are able to manage the AID system, then they should also have easy access and proximity to the AID system intraoperatively. The use of an AID system during surgery is not recommended if the insulin requirements are expected to fluctuate significantly intraoperatively. In that case intravenous insulin delivery with insulin dosing software instead of subcutaneous insulin delivery would be more appropriate with either an intravenous or subcutaneous glucose sensor. AID systems can be continued during the operation if there are no concerns regarding device malfunction. However, there are no good data available on the safety or maximum safe duration of closed-loop control during anesthesia. Even with control by an AID system, BG concentrations should be monitored intraoperatively.
Recommendations for Continuation of AID Systems in the Hospital
Clinical Practice
Strong Recommendations
- HCPs should prescribe AID systems only for appropriate candidates, who will need to have adequate knowledge and skills for using AID systems.
- HCPs should reassess a decision periodically to transition use of outpatient AID systems into the hospital in order to ensure that AID system continues to represent the best treatment option for each patient.
- HCPs should prepare an alternative plan for diabetes management in case it becomes inappropriate for a patient to continue using an AID system in the hospital.
- HCPs should discontinue AID systems in critically ill hospitalized patients (such as those with hypovolemia or sepsis).
- HCPs should recognize glycemic patterns due to CGM compression, which can cause false low readings.
Mild Recommendation
- HCPs should avoid initiating an AID system during a hospitalization.
Research
Strong Recommendations
- Researchers need to conduct studies about whether continuing AID systems in the hospital is beneficial to improve glycemic management or clinical outcomes.
- Researchers need to provide data on hospital outcomes when using AID systems in the hospital, including improved glycemic outcomes, detection and/or reduction of hypoglycemia, reduction of ICU LOS, and cost-effectiveness.
- Manufacturers need to research whether all types of CGMs and AID systems can be used during radiological/imaging studies or diathermy.
Hospital Policies
Strong Recommendations
- Hospitals need to develop institution-specific protocols and order sets for the proper use of AID systems during a hospitalization.
- Hospitals need to require that patients using AID systems bring with them sufficient supplies for these devices during a hospitalization.
- Hospitals need to develop protocols for using AID systems during elective procedures and surgeries.
Recommendation Not Reaching Consensus
- HCPs should switch AID systems from “auto” mode to “manual” mode when a patient is admitted to the hospital wearing an AID system.
Logistics and Hands-On Care of Hospitalized Patients Using CGMs and AID Systems
Chair: Suzanne Lohnes, MA, RN, CDCES, CPT
University of California San Diego Medical Center, La Jolla, CA, USA
Potential Opportunities
Expectations for Patients and Hospital Staff and Practical Considerations for Use of CGMs and AID Systems in the Acute Care Setting
Continuation of CGM use can be a helpful adjunct to management in the acute care setting and can increase patient satisfaction. However, because CGMs are not currently cleared by FDA for the inpatient environment, a policy addressing practical considerations for use of CGMs and AID systems in hospitalized patients is needed.
Potential Barriers
Necessary Hospital Responsibilities
It is important that key tasks, roles, and responsibilities, related to work system domains (technology/data, tasks, personnel, structure/organization, and environment), are addressed for safe and effective implementation.132 Below are listed potential responsibilities delineated by team members. It is helpful for diabetes team members to be interchangeable (eg, subspecialty consultant with pharmacist or nurse with patient care technician). Furthermore, it is appropriate to predefine tasks, person assignments, policies, procedures, and a clear organizational structure (eg, determination of committee reporting) around monitoring and interpretation of data, to facilitate use of CGMs and AID systems.
Necessary Patient Responsibilities
Patients who wish to continue use of CGMs or AID systems in the acute care setting should read a detailed set of information and should review and sign a patient agreement about hospital policy. The panel developed a sample patient agreement for the use of CGMs or AID systems in the hospital presented in Figure 1. This agreement is meant to be an example for a subcutaneous non-implanted sensor. Each institution must develop their own agreement and they should consider manufacturer labeling.

Figure 1. Continuous glucose monitors or automated insulin dosing system sample patient agreement. CGM, continuous glucose monitoring; CT, computed tomography; MRI, magnetic resonance imaging.
CGMs may be used for guidance about the direction and magnitude of changes in glucose concentrations. The patient should notify hospital staff if they are observing glucose excursions out of range or if they experience symptoms of hypoglycemia. The patient should bring all supplies (infusion sets, sensors, receiver, and so on) needed for continuation of home use for the duration of a hospitalization and be responsible for maintenance of their device and changing sites as directed during a hospitalization. Device supplies may be stored per hospital policy and will be returned to the patient upon discharge.
Necessary HCP Responsibilities
Inpatient caregivers must (1) confirm that it is appropriate for a patient to continue using a CGM or an AID system, (2) discuss hospital policy with the patient, and (3) review an agreement with the patient. After the patient agreement is signed, the HCP should place an order for inpatient use of a CGM or an AID system. A patient’s ability to safely continue use of a CGM or an AID system (which may change during the hospitalization) must be regularly assessed by nursing staff and HCPs.133 Daily documentation per institutional policy will be needed throughout the hospitalization. If there is concern for patient’s ability to use a CGM or an AID system, then the caregiver will recommend an alternative treatment plan.
Necessary Nursing Responsibilities
In collaboration with other inpatient HCPs, it is important for nursing to assess the patient’s suitability for using a CGM or an AID system and review hospital policies with the patient. It is also important for nursing to assess the insertion site and document site changes in the EHR.
Treatment decisions based on CGM data linked to insulin dosing software might lead to unwanted outcomes unless the safety and efficacy of the system in the acute care setting can be clearly established. For patients using AID systems in the hospital who are going to be transitioned to and/or discharged with subcutaneous multiple-dose insulin therapy, if the insulin dosing information (from “auto” mode) is not available in the EHR, then an estimate of insulin requirements might be inaccurate and could lead to dysglycemia following discharge.
Standard approaches to documentation are also needed. The panel recognized a spectrum of practice for nursing documentation and institutional requirements. Nursing should document all AID system device settings, including any insulin boluses in “manual” mode, in the inpatient progress notes and/or in the patient’s bedside log, which is scanned into the EHR. Additionally, the frequency that this information is documented (ie, every shift vs daily) may vary based on individual hospital resources and policies.
Specialty Consultation
When using CGMs or AID systems in the acute care setting, specialty consultation, if available, is required and the request for consultation should be documented. While some institutions have inpatient diabetes support available for in-person consultation and ongoing management, the panel recognizes there are circumstances in which inpatient diabetes expertise may not be readily available. The panel suggested consideration for telemedicine consultation with a diabetes specialist if necessary. It is useful to document the patient’s ability to use the technology to assist with glucose management.
Recommendations for Logistics and Hands-On Care of Hospitalized Patients Using CGMs and AID Systems
Clinical Practice
Strong Recommendations
- HCPs should inquire about and document the medication and supplement history of patients who use CGMs to determine whether there are any agents that can interfere with glucose measurements.
- HCPs should ensure that off-label use of CGMs and AID systems is consistent with medical practice and appropriate precautions have been taken to protect patients.
- Nursing should document hands-on training of CGM use and AID system therapy through a technology certification program.
- Nursing should confirm that the patient is appropriate to continue using a CGM or an AID system and also review the agreement and hospital policy with the patient.
- Nursing should inspect the insertion site every shift with attention to skin integrity and signs of erythema or infection, and should document site changes.
- Nursing should know device basics, institutional policies, HCPs’ roles, and whom to contact if questions arise.
- Nursing should administer a patient competency assessment or survey to assess patient ability to safely assist with managing a CGM or an AID system.
- Nursing should set expectations and clarify that there will be a need to continue checking POC capillary glucose even when using a CGM.
- Nursing should measure POC BG concentrations to confirm or supplement CGM readings (usually a minimum of four times daily: before each of three meals and at bedtime if patients are eating, or every six hours if patients are fasting) as well as at patient request; however, the CGM glucose, trend arrows, and rate of change may be used to help determine if and when a BG test is required.
Research
Strong Recommendations
- Researchers need to conduct further studies on the best logistics and hands-on care for patients using CGMs and AID systems to achieve the best outcomes.
- Manufacturers need to research interoperable components for AID systems that are compatible with hospital EHRs.
Hospital Policies
Strong Recommendations
- Hospitals need to provide interpreter services to translate CGM and AID system agreements.
- Hospitals need to state in their policy and patient agreement documents that treatment decisions will be based on hospital-calibrated BGM readings (or laboratory readings) and not on CGM readings, barring a need to isolate a patient with a severe and highly contagious infection.
- Hospitals need to maintain their CGM and AID system policy and patient agreement documents in easily accessible electronic files stored in the EHR order set for CGMs and AID systems.
- Hospitals need to develop policies for when to discontinue or temporarily suspend the use of CGMs and AID systems
- Hospitals need to survey their HCPs, nursing, and patients to improve outcomes and satisfaction.
Data Management of CGMs and AID Systems in the Hospital
Chair: James H. Nichols, PhD, DABCC, FAACC
Vanderbilt University Medical Center, Nashville, TN, USA
Potential Opportunities
Policies and Procedures
As previously noted, there is a distinction between CGM glucose values and laboratory glucose values, and CGM data are currently not part of the laboratory information system. Rather, CGM data are analogous to ICU vital sign monitoring data rather than lab values like serum potassium and sodium. Because of this distinction, it is important to consider where in the medical records these data should reside and how they should be displayed, such as in reports, tables, or graphs. Given this known difference between CGM glucose values and lab glucose values,134 criteria should also be developed on when to check or cross-reference CGM values with a POC or laboratory glucose test. A related question is whether or not clinical decisions should be made on the basis of CGM data, or whether clinicians should always obtain a laboratory or POC glucose test for treatment decision-making. Finally, criteria should be established as to whether a minimum number of laboratory or POC BG tests must be performed while patients are using CGMs or AID systems in the hospital. Manufacturers of some CGMs have recommended a calibration frequency, but those recommendations are intended for outpatient use, and might not be adequate for inpatient use.
As part of the standardization of summary metrics, we should also develop clear criteria for values or trends that require a clinical intervention. The panel discussed creating a framework for clinical action based on CGM data. This includes understanding what data and trends are actionable, as well as what the appropriate clinical interventions might be. Critical values are considered to be imminently life-threatening test results that require immediate contact by the ordering HCPs. CGMs can trend the rise and fall of glucose concentrations, and can predict critical hypo- or hyperglycemia. Data management systems can be set to alarm when CGM glucose trends reach or cross certain critical values. These alarms should lead to clinician and patient notification so that appropriate actions may be taken in a timely fashion.
The panel noted that data and security are major concerns in Germany and the rest of Europe. In Europe, every manufacturer uses a different data scheme and interface to download their data, which can be confusing.
Information Technology Infrastructure
The Health Insurance Portability and Accountability Act of 1996 (HIPAA) protects health information, promotes transparency, trust, and patient welfare in medical practice. Since CGMs and AID systems collect protected health information (PHI), when they are used by institutions and clinicians to make medical decisions, institutions have a responsibility to treat it like all other PHI, meaning they must ensure the integrity, security, and appropriate availability of that data. Documenting CGM results and data in the EHR designates it as part of the medical record, and it becomes subject to HIPAA. The Information Technology (IT) department is needed to assist with licenses to download the data, and install the software into each hospital system.
Healthcare facilities should adopt the Unique Device Identifier (UDI) system to track devices in the EHR. In 2013, the FDA issued guidelines for the implementation of a global UDI system to adequately identify and track medical devices across their lifecycle, from distribution to patient use.135 The UDI final rule established a timeline for all qualifying medical devices in the United States to be compliant with UDI labeling by 2022.136 Diabetes technologies like BGMs, CGMs, CSII systems, and AID systems are all required to bear a UDI. Institutions should rapidly move toward UDI adoption and integration into the EHR, and ensure that CGM and AID system data are associated with the correct UDI for safety and quality assurance.
Data
Panelists recognized that there is limited evidence on how CGM data are integrated into EHRs at this time. With the near-universal adoption of EHRs among inpatient facilities in the United States,137 integrating device data into the EHR is important for quality and consistency. Several groups have explored the integration of these data into the EHR,138–140 but many questions still remain regarding best practices for the acquisition, storage, display, and use of those data.
Distinctions should be made when recording CGM data in the EHR, since CGM data differ from laboratory glucose results. CGMs measure glucose within interstitial fluid, while laboratory instruments measure glucose in plasma, serum, or whole blood. This means that CGM data may not agree with laboratory glucose measurements collected at the same time.134 While individual CGM data points may be less precise than lab instrumentation-generated values, a major advantage offered by CGMs is the presentation of multiple data points over time. These create an opportunity to evaluate glucose patterns as well as trends in the rate of change, percent of time spent hypo- or hyperglycemic or within target range, and estimate stability/instability of the glucose concentration over time. These summary patterns may be more valuable than individual data points and provide a synthesis of the patient’s overall glycemic status.
Data Patterns
As EHR integrations of CGM data become more common, HCPs with a wider variety of backgrounds in training and experience with CGM data interpretation will have access to this data. Some might be less familiar with its use and interpretation. It is important that standardized, clear, and interpretable summary metrics be established in order to facilitate the clinical use of CGM data in the hospital setting.
When considering how to integrate device data, the first decision is how to source data. There are two main options: (1) obtaining the data directly on a platform provided by the manufacturer (eg, Abbott, Dexcom, or Medtronic) and (2) obtaining the data from a third-party aggregator, eg, Tidepool (Tidepool, Palo Alto, CA, USA) or Glooko (Glooko, Inc., Mountain View, CA, USA). Each of these approaches has advantages and disadvantages, as well as associated costs and technical requirements. It may be reasonable to use a hybrid approach, connecting directly with a few manufacturers that have significant market share, and then using an aggregator to capture other devices.
The next decision is what data to extract. There are several options for extracting, storing, and displaying CGM data, and at varying levels of complexity (Table 8). Static reports (view-only documents, typically PDFs) are the simplest, and some CGM manufacturers have already developed mechanisms to bring the CGM reports found on their provider platforms into the EHR. Structured summary data are predefined and standardized, and can be added to existing data tables in the EHR for charting, trending, and so on. Structured continuous data refer to the hundreds of daily individual BG measurements, and are the most complex to manage, but potentially offer the most flexibility and control.
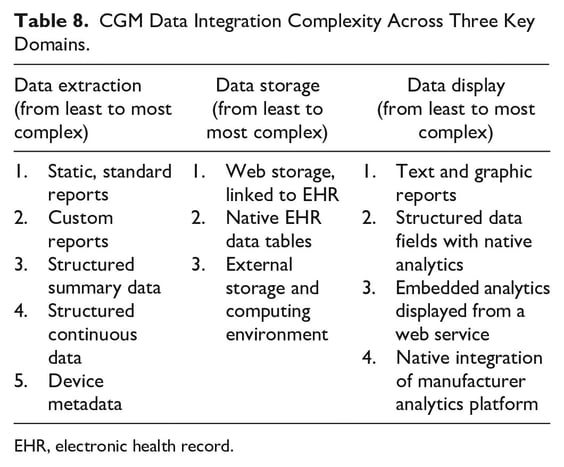
Data storage and display will be dictated by the type of data extracted from the device. Reports and structured summary data can be stored in native EHR data tables, but continuous glucose readings would likely overwhelm those tables, and would best be stored in a separate environment. In terms of displaying the data, this can be accomplished in a variety of ways described in Table 8.
A consensus list of core data elements should be developed and standardized across all models and manufacturers. Data standards and ontologies are critical for ensuring interoperability across information systems.141 A core set of data elements and definitions developed and applied by the entire CGM industry would facilitate storage and use of CGM data. Finally, core data elements would ideally be submitted to the appropriate governing bodies for inclusion in existing healthcare ontologies and common data models, such as Systematized Nomenclature of Medicine—Clinical Term, Logical Observation Identifiers Names and Codes, and Observational Medical Outcomes Partnership.
Patient-reported outcomes (PROs) are any reports of the status of a patient’s health condition that come directly from the patient, without interpretation of the patient’s response by a clinician or anyone else.142 PROs can be leveraged for research, clinical care, and quality improvement. While several groups are actively working on the development of PROs in diabetes, there is still significant work to be done.143 The development, dissemination, and implementation of diabetes technology-specific PROs will enable a more holistic approach to patient care and research.
Atypical Scenarios
Guidelines should address the use of CGMs and AID systems for diagnoses other than diabetes, where glucose monitoring is valuable. In pediatrics, several clinical situations require close monitoring of BG concentrations and tight glycemic control, such as the titration of glucose infusion rates in premature infants on total parenteral nutrition. Early detection of hypoglycemia in infants with inborn errors of metabolism (eg, fatty acid oxidation disorders, ketotic hypoglycemic disorders, and disorders of gluconeogenesis) could be another critical use for CGMs in the hospital setting. In these diseases, infants are often allowed to become hypoglycemic as a challenge in order to draw critical diagnostic labs. CGM measurements could make that process less stressful for parents and HCPs and safer for patients.
Economic Analysis
Panelists had concerns with the costs of some CGMs and AID systems being a limiting factor (ie, batteries, sensors, transmitters, and/or a monitor or smartphone), but found that some CGMs are affordable. Panelists considered questions about the reimbursement for these devices. Who is responsible for covering their costs and consumable components? What if the patient has a device from one manufacturer, but the hospital only stocks supplies from a different manufacturer? Panelists also discussed the economic implications of CGM and AID system use in hospitalized patients. Inpatient hypo- and hyperglycemia, which might prove to be reduced with structured CGM or AID system programs, have been associated with increased LOS, readmissions, and costs.48,144 In patients undergoing cardiac surgery, studies suggested potential cost saving with intensive glycemic management (targeting 100-140 mg/dL).145 Finally, panelists acknowledged the need for well-powered studies comparing the use of CGMs vs POC BGMs on hospitalization costs.146
Potential Barriers
Regulatory Considerations
The Clinical and Laboratory Improvement Amendment of 1988 (CLIA) sets a minimum quality standard for any laboratory test performed in the United States for patient care or clinical decision making. Externally attached patient-dedicated monitoring devices like pulse oximetry capnography are not subject to CLIA.147 CGMs and AID systems are also automatic monitoring devices that are wearable and continuously or intermittently detect glucose concentrations in interstitial fluid or tissue fluid. There is no sample collection and analysis in a separate instrument that can be calibrated or validated with a quality control sample. As such, a CGM is more of a monitoring device than a laboratory instrument, and should not be subject to CLIA.
Although CGMs and AID systems should not be subject to CLIA, quality control is still an important consideration for inpatient CGM and AID system use. Previous consensus panels have stressed the need for clear safety and quality protocols to be in place.1 There is known variation between sensors, both between brands and within brands. Also, calibration errors can lead to significant deviations in glucose values. Currently, some hospitals using CGMs require a patient agreement, which outlines that the patient can still use their CGM, but hospital BGM testing is still mandatory. See Figure 1 for a sample agreement. In Germany, laboratory quality control guidelines require twice-daily internal testing and quarterly external testing for hospital lab meters.148 This is a prerequisite for the use of data for diagnostic or therapeutic decisions. With CGMs, there is no sample and no control materials, so these procedures cannot be applied to CGMs, which is why some BG monitoring is still mandatory in the hospital. One possible path forward is for manufacturers to develop a mechanism to perform quality control procedures for CGMs. Otherwise, CGMs in the hospital may be limited to adjunctive use only.
Off-label use of prescription drugs and devices is common in modern medical practice, and has been recognized as “an accepted and necessary corollary of the FDA’s mission to regulate in this area without directly interfering with the practice of medicine” by the United States Supreme Court.149 A manufacturer may not market unapproved uses of a medical device, but a physician may in their independent judgment decide to use a cleared device in an off-label manner. While off-label use is seen as accepted practice, it does not shield physicians from liability, and there is potential tort exposure. Whether a hospital would also be liable under those circumstances would probably depend on what sort of control it exerted over the physician. If it is for an employed physician, then the hospital might be liable for the physician’s actions under a theory of respondeat superior, which is a doctrine that states that an employer is responsible for the acts of an employee. If the physician is an independent contractor, then hospital liability for the physician’s actions would be more difficult to establish. One way to evaluate the liability or legal risk of off-label use is to consider whether or not that action may expose the practitioner to a claim of negligence or malpractice. Negligence can be thought of as a breach in duty (eg, to a patient), or as the failure to act reasonably in light of foreseeable consequences.
Data Privacy and Security
Another potential risk is around the data itself, and whether they are being stored and protected with the proper precautions for PHI. Overall, this should not be seen as an obstacle, provided it is consistent with standard practice. Tracking UDIs may also be an appropriate risk-mitigation step that can address some safety and quality concerns. Software whose sole purpose is to store and summarize data may not be considered a medical device, but there are still privacy and cyber-security concerns with these products.150,151 Document retention policies are important in order to protect HCPs and hospitals from possible legal actions. In situations where the hospital is developing custom institutional (“home-brewed”) software, it is important to follow cybersecurity risk management standards and realize that not all insurance policies cover cyber security breaches related to custom-developed software. Risk management teams should be in close communication with their insurance brokers to ensure appropriate coverage for that type of activity.
Finally, it may be important to develop maturity models for diabetes technology. Maturity models are tools developed in the information technology field to provide guidance to organizations for assessing their current level of development in a particular topic, as well as a roadmap for systemic and structured improvement.152 Healthcare IT maturity models have been developed to cover a variety of topics, ranging from continuity of care and healthcare analytics, to telemedicine and mobile technology.153 Diabetes technology integration would greatly benefit from a maturity model to help guide implementation at healthcare institutions in a systematic way.
Recommendations for Data Management of CGMs and AID Systems in the Hospital
Clinical Practice
Strong Recommendation
- HCPs should develop a set of core data elements and definitions for CGM data for inclusion in common data models and the EHR.
Mild Recommendation
- Nursing should contact an HCP immediately when CGM results cross critical value thresholds set by the institution.
Research
Strong Recommendations
- Researchers need to conduct further studies on the best data management practices of CGMs and AID systems.
- Researchers need to develop and validate robust glucose telemetry systems for both ICU and non-ICU populations.
- Researchers need to develop a diabetes technology maturity model that helps institutions understand the requirements to successfully integrate diabetes-related data and technology.
- Researchers need to develop, disseminate, and validate CGM- and AID system-specific PRO measures to improve patient care.
- Manufacturers need to research methods for quality control for CGMs and AID systems, which is critical as part of inpatient use of CGMs and AID systems.
- Manufacturers need to research optimally expanded device labeling in order to overcome clinical inertia and align practice with regulatory policy.
- Manufacturers need to research systems for integration of CGM data following initial upload into the cloud (eg, the Eversense CGM) subsequently into the EHR.
- Manufacturers need to research secure communication systems for protecting data from wireless wearables, telemedicine systems, and Bring-Your-Own-Device portable computers used by HCPs (also known as “data in motion”).
Mild Recommendation
- Researchers need to develop computerized insulin decision support system that will integrate with CGMs.
Hospital Policies
Strong Recommendations
- Hospitals need to develop appropriate security protocols, dedicated data storage, visualization tools, and adequate cyber insurance coverage (also known as “data at rest”).
- Hospitals need to integrate AID system data into the EHR system for nursing and HCPs to have easy access to this information.
- Hospitals need to determine the number of laboratory or POC BG tests that must be performed while patients are using CGMs or AID systems in the hospital.
- Hospitals need to adopt the UDI system for healthcare facilities to track devices in the EHR.
- Hospitals need to identify CGM data reports in the patient’s EHR to distinguish them from laboratory glucose results.
- Hospitals need to present clear criteria to clinicians to identify data that will require intervention.
- Hospitals need to implement CGM- and AID system-specific PROs to improve patient care.
- Hospitals need to develop a universal platform for their EHRs that can be used by all CGMs to present core data elements, summary glucometrics, consistent formats, and uniform interfaces across all CGM products.
- Hospitals need to arrange for CGM results to be automatically uploaded into the EHR.
- Hospitals need to manage CGM data with the same safety and security measures as all other PHI.
- Hospitals need to develop policies for CGM and AID system use with atypical scenarios outside of diabetes, when glucose monitoring is valuable.
Conclusion
This consensus guideline for subcutaneous CGMs and AID systems was created to provide recommendations to clinicians, researchers, and hospitals for promoting the safe and effective use of CGMs and AID systems in the hospital environment. Through a consensus process, an international expert panel voted on 78 recommendations. Seventy-seven of the recommendations were classified as either strong or mild, and one failed to reach consensus (Table 9). The panel’s recommendations are intended to support clinical practice, future research, and improved hospital policies, to facilitate the use of these tools. The success of this guideline will be the impact to clinicians, researchers, manufacturers, and hospitals in the management of hospitalized patients with diabetes.

Acknowledgements
The authors thank the following meeting attendees for their contributions to the ideas summarized in this report: Leslie Landree and Courtney Lias (FDA). We would also like to acknowledge attendees Irina Nayberg (Mills-Peninsula Medical Center), Robby Booth (Glytec), Daniel Chernavsky (Dexcom), Jeff Draper (Abbott Diabetes Care), Corinne Fantz (Roche Diagnostics), Andrew S. Rhinehart (Medtronic), Robert A. Vigersky (Medtronic), Nauni Virdi (Abbott Diabetes Care), and Tomas C. Walker (Dexcom). The College of American Pathologists was represented by David B. Sacks, Endocrine Society was represented by Amisha Wallia, and the Association of Diabetes Care and Education Specialists was represented by Dessa Garnett Awadjie. We thank Jeffrey Joseph for providing background information. Finally, we thank Annamarie Sucher for her expert editorial assistance.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RJG is supported in part by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institute of Health under Award Number 1K23DK123384-01 and P30DK11102. RJG received research support (to Emory University) for investigator-initiated studies from Novo Nordisk and Dexcom, and consulting fees from Abbott Diabetes Care, Eli Lilly, Sanofi, Novo Nordisk, and Valeritas. GEU is partly supported by research grants from the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002378 from the Clinical and Translational Science Award program and a National Institutes of Health (NIH) grant U30, P30DK11102, and has received research grant support to Emory University for investigator-initiated studies from Sanofi, Novo Nordisk, and Dexcom. AB received grant support through NIDDK 085516 and 106785. JHN received research grant support and professional speaking honoraria from Abbott and Roche Diagnostics. EKS was partially supported by the VA MERIT award (#1I01CX001825) from the United States Department of Veterans Affairs Clinical Sciences Research and Development Service. EKS has received unrestricted research support from Dexcom (to Baltimore VA Medical Center and to University of Maryland) for the conduction of clinical trials. JE’s efforts were supported by the Food and Drug Administration under award number P50FD006425 for The West Coast Consortium for Technology & Innovation in Pediatrics. The funding source had no involvement in the development of this manuscript or in the decision to submit the paper for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the FDA. BB is on medical advisory boards for Convatec, Medtronic, and Tolerion. GF is general manager and medical director of the Insitute for Diabetes Technology (Institut für Diabetes-Technologie Forschungs- und Entwicklungsgesellschaft mbH an der Universität Ulm, Ulm, Germany), which carries out clinical studies on the evaluation of BG meters, with CGM systems and medical devices for diabetes therapy on its own initiative and on behalf of various companies. GF has received speakers’ honoraria or consulting fees from Abbott, Ascensia, Dexcom, i-SENS, LifeScan, Menarini Diagnostics, Metronom Health, Novo Nordisk, PharmaSense, Roche, Sanofi, Sensile, and Ypsomed. RH reports having received speaker honoraria from Eli Lilly and Novo Nordisk, serving on advisory panel for Eli Lilly and Novo Nordisk; receiving license fees from BBraun and Medtronic; having served as a consultant to BBraun, patents related to closed-loop insulin delivery, and director at CamDiab. DNO has served on advisory boards for Abbott, Medtronic, MSD, Novo, Roche, and Sanofi; received research support from Medtronic, Novo, Roche, Lilly, and Sanofi; and travel support from Novo and MSD. AW received research support from United Health Group and Eli Lilly, and received research funding from Novo Nordisk. DCK is a consultant to Dexcom, Eoflow, Fractyl, Lifecare, Novo Nordisk, Roche Diagnostics, and Thirdwayv. RJR, SL, NEP, DGA, LB, CBC, LH, NM, TN, MR, DBS, JJS, TS, JYZ, and JH have nothing to disclose.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by grants from Abbott Diabetes Care, Dexcom, and Medtronic.
References
| 1. | Wallia, A, Umpierrez, GE, Rushakoff, RJ, et al. Consensus statement on inpatient use of continuous glucose monitoring. J Diabetes Sci Technol. 2017;11(5):1036–1044. Google Scholar | SAGE Journals
|
| 2. | Dexcom Continuous Glucose Monitoring . Hospital use during the COVID-19 pandemic. Dexcom. April 1, 2020. https://www.dexcom.com/hospitalcovid-19. Accessed September 10, 2020. Google Scholar
|
| 3. | Abbott’s FreeStyle ® Libre 14 day system now available in U.S. for hospitalized patients with diabetes during COVID-19 pandemic . Abbott MediaRoom. April 8, 2020. https://abbott.mediaroom.com/2020-04-08-Abbotts-FreeStyle-R-Libre-14-Day-System-Now-Available-in-U-S-for-Hospitalized-Patients-with-Diabetes-During-COVID-19-Pandemic. Accessed September 10, 2020. Google Scholar
|
| 4. | Galindo, RJ, Aleppo, G, Klonoff, DC, et al. Implementation of continuous glucose monitoring in the hospital: emergent considerations for remote glucose monitoring during the COVID-19 pandemic. J Diabetes Sci Technol. 2020;14(4): 822–832. doi:10.1177/1932296820932903. Google Scholar | SAGE Journals
|
| 5. | Klonoff, DC, Perz, JF. Assisted monitoring of blood glucose: special safety needs for a new paradigm in testing glucose. J Diabetes Sci Technol. 2010;4(5):1027–1031. Google Scholar | SAGE Journals
|
| 6. | Korytkowski, M, Antinori-Lent, K, Drincic, A, et al. A Pragmatic approach to inpatient diabetes management during the COVID-19 pandemic. J Clin Endocrinol Metab. 2020;105(9). Google Scholar | Crossref | Medline
|
| 7. | Gujral, UP, Johnson, L, Nielsen, J, et al. Preparedness cycle to address transitions in diabetes care during the COVID-19 pandemic and future outbreaks. BMJ Open Diabetes Res Care. 2020;8(1): 1–7. Google Scholar | Crossref
|
| 8. | Didyuk, O, Econom, N, Guardia, A, Livingston, K, Klueh, U. Continuous glucose monitoring devices: past, present, and future focus on the history and evolution of technological innovation [Published online January 13, 2020]. J Diabetes Sci Technol. doi:10.1177/1932296819899394. Google Scholar | SAGE Journals
|
| 9. | Galindo, RJ, Migdal, AL, Umpierrez, GE. Are we ready to move beyond capillary glucose testing and insulin injections? Am J Med Sci. 2019;358(5):315–316. Google Scholar | Crossref | Medline
|
| 10. | Beyond A1C Writing Group . Need for regulatory change to incorporate beyond A1C glycemic metrics. Diabetes Care. 2018;41(6):e92–e94. Google Scholar | Crossref | Medline
|
| 11. | Basu, A, Dube, S, Veettil, S, et al. Time lag of glucose from intravascular to interstitial compartment in type 1 diabetes. J Diabetes Sci Technol. 2015;9(1):63–68. Google Scholar | SAGE Journals
|
| 12. | International Biomedical . GlucoScout- fully automated blood glucose sampling and measurement. July 2010. https://www.int-bio.com/wp-content/uploads/2016/06/gluco-scout-brochure.pdf. Accessed September 10, 2020. Google Scholar
|
| 13. | OptiScan Corp . OptiScanner 5000 – Automated bedside glucose monitoring for critical care. OptiScan. 2018. https://optiscancorp.com/technology-products/optiscanner-5000/. Accessed September 10, 2020. Google Scholar
|
| 14. | Bochicchio, GV, Hipszer, BR, Magee, MF, et al. Multicenter observational study of the first-generation intravenous blood glucose monitoring system in hospitalized patients. J Diabetes Sci Technol. 2015;9(4):739–750. Google Scholar | SAGE Journals
|
| 15. | Crane, BC, Barwell, NP, Gopal, P, et al. The development of a continuous intravascular glucose monitoring sensor. J Diabetes Sci Technol. 2015;9(4):751–761. Google Scholar | SAGE Journals
|
| 16. | Schierenbeck, F, Öwall, A, Franco-Cereceda, A, Liska, J. Evaluation of a continuous blood glucose monitoring system using a central venous catheter with an integrated microdialysis function. Diabetes Technol Ther. 2013;15(1):26–31. Google Scholar | Crossref | Medline | ISI
|
| 17. | Kosiborod, M, Gottlieb, RK, Sekella, JA, et al. Performance of the Medtronic Sentrino continuous glucose management (CGM) system in the cardiac intensive care unit. BMJ Open Diabetes Res Care. 2014;2(1):e000037. Google Scholar | Crossref | Medline
|
| 18. | Tripyla, A, Herzig, D, Joachim, D, et al. Performance of a factory-calibrated, real-time continuous glucose monitoring system during elective abdominal surgery. Diabetes Obes Metab. 2020;22(9):1678–1682. doi:10.1111/dom.14073. Google Scholar | Crossref | Medline
|
| 19. | Migdal, AL, Spanakis, EK, Galindo, RJ, et al. Accuracy and precision of continuous glucose monitoring in hospitalized patients undergoing radiology procedures. J Diabetes Sci Technol. 2020;14(6):1135–1136. doi:10.1177/1932296820930038. Google Scholar | SAGE Journals
|
| 20. | Thomas, C, Welsh, JB, Lu, S, Gray, JM. Safety and functional integrity of continuous glucose monitoring components after simulated radiologic procedures [Published online April 22, 2020]. J Diabetes Sci Technol. doi:10.1177/1932296820920948. Google Scholar | SAGE Journals
|
| 21. | Takatsu, Y, Shiozaki, T, Miyati, T, Asahara, M, Tani, Y. Are the recorded data of flash glucose monitoring systems influenced by radiological examinations? Radiol Phys Technol. 2019;12(2):224–229. doi:10.1007/s12194-019-00505-x. Google Scholar | Crossref | Medline
|
| 22. | FreeStyle Libre . 14-day System | Glucose Sensor & Reader | FreeStyleLibre.us. https://www.freestylelibre.us/system-overview/freestyle-14-day.html. Accessed September 10, 2020. Google Scholar
|
| 23. | FreeStyle Libre . 2-best glucose monitor for kids – coming soon for patients 4+. https://www.freestylelibre.us/system-overview/freestyle-libre-2.html. Accessed September 10, 2020. Google Scholar
|
| 24. | Dexcom G6 Continuous Glucose Monitoring System . Dexcom. February 15, 2018. https://www.dexcom.com/g6-cgm-system. Accessed September 10, 2020. Google Scholar
|
| 25. | Medtronic Guardian Sensor 3 . August 30, 2018. http://www.medtronicdiabetes.com/products/guardian-sensor-3. Accessed September 10, 2020. Google Scholar
|
| 26. | Eversense Continuous Glucose Monitoring | Long-term Continuous Glucose Monitor . 2019. https://www.eversensediabetes.com/. Accessed September 10, 2020. Google Scholar
|
| 27. | Galindo, RJ, Beck, RW, Scioscia, MF, Umpierrez, GE, Tuttle, KR. Glycemic monitoring and management in advanced chronic kidney disease. Endocr Rev. doi:10.1210/endrev/bnaa017. Google Scholar | Crossref
|
| 28. | FreeStyle Libre . User’s Manual. 2019. https://freestyleserver.com/Payloads/IFU/2017_dec/ART34745-107_rev-A-WEB.pdf. Accessed September 10, 2020. Google Scholar
|
| 29. | FreeStyle Libre 2 User’s Manual. 2020. https://freestyleserver.com/Payloads/IFU/2020/q2/ART40703-001_rev-D-Web.pdf. Accessed September 10, 2020. Google Scholar
|
| 30. | Alva, S, Bailey, T, Brazg, R, Budiman, ES, Castorino, K, Christiansen, MP, Forlenza, G, Kipnes, M, Liljenquist, DR, Liu, H. Accuracy of a 14 day factory calibrated continuous glucose monitoring system with advanced algorithm in pediatric and adult population with diabetes. J Diabetes Sci Technol. 2020. Google Scholar
|
| 31. | Calhoun, P, Johnson, TK, Hughes, J, Price, D, Balo, AK. Resistance to acetaminophen interference in a novel continuous glucose monitoring system. J Diabetes Sci Technol. 2018;12(2):393–396. Google Scholar | SAGE Journals
|
| 32. | Dexcom G6 System User Guide. March 2020. https://s3-us-west-2.amazonaws.com/dexcompdf/G6-CGM-Users-Guide.pdf. Accessed September 10, 2020. Google Scholar
|
| 33. | Bliss, C, Huang, E, Savaser, D. Safety of a continuous glucose monitoring device during hyperbaric exposure. Undersea Hyperb Med. 2020;47(1):13–19. Google Scholar | Medline
|
| 34. | Christiansen, MP, Garg, SK, Brazg, R, et al. Accuracy of a fourth-generation subcutaneous continuous glucose sensor. Diabetes Technol Ther. 2017;19(8):446–456. Google Scholar | Crossref | Medline
|
| 35. | Guardian Sensor 3 User Guide. 2018. https://www.medtronicdiabetes.com/sites/default/files/library/download-library/user-guides/Guardian-Sensor-3-user-guide.pdf. Accessed September 10, 2020. Google Scholar
|
| 36. | Lorenz, C, Sandoval, W, Mortellaro, M. Interference assessment of various endogenous and exogenous substances on the performance of the eversense long-term implantable continuous glucose monitoring system. Diabetes Technol Ther. 2018;20(5):344–352. Google Scholar | Crossref | Medline
|
| 37. | Eversense user guide. June 4, 2019. https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160048S006c.pdf. Accessed September 10, 2020. Google Scholar
|
| 38. | Fokkert, MJ, Damman, A, van Dijk, PR, et al. Use of FreeStyle libre flash monitor register in the Netherlands (FLARE-NL1): patient experiences, satisfaction, and cost analysis. Int J Endocrinol. 2019;2019:4649303. Google Scholar | Crossref | Medline
|
| 39. | Vesco, AT, Jedraszko, AM, Garza, KP, Weissberg-Benchell, J. Continuous glucose monitoring associated with less diabetes-specific emotional distress and lower A1c among adolescents with type 1 diabetes. J Diabetes Sci Technol. 2018;12(4):792–799. Google Scholar | SAGE Journals
|
| 40. | Shehav-Zaltzman, G, Segal, G, Konvalina, N, Tirosh, A. Remote glucose monitoring of hospitalized, quarantined patients with diabetes and COVID-19. Diabetes Care. 2020;43(7):e75–e76. Google Scholar | Crossref | Medline
|
| 41. | Beck, RW, Riddlesworth, TD, Ruedy, K, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections. Ann Intern Med. 2017;167(6):365–374. Google Scholar | Crossref | Medline
|
| 42. | Klonoff, DC, Kerr, D. A Simplified approach using rate of change arrows to adjust insulin with real-time continuous glucose monitoring. J Diabetes Sci Technol. 2017;11(6):1063–1069. Google Scholar | SAGE Journals
|
| 43. | Singh, LG, Satyarengga, M, Marcano, I, et al. Reducing inpatient hypoglycemia in the general wards using real-time CGM- the Glucose Telemetry system: a randomized clinical trial [published online ahead of print August 5, 2020]. Diabetes Care. doi:10.2337/dc20-0840. Google Scholar | Crossref
|
| 44. | Feig, DS, Donovan, LE, Corcoy, R, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet. 2017;390(10110):2347–2359. Google Scholar | Crossref | Medline
|
| 45. | Polsky, S, Garcetti, R. CGM, Pregnancy, and remote monitoring. Diabetes Technol Ther. 2017;19(S3):S49–S59. Google Scholar | Crossref | Medline
|
| 46. | Ancona, P, Eastwood, GM, Lucchetta, L, Ekinci, EI, Bellomo, R, Mårtensson, J. The performance of flash glucose monitoring in critically ill patients with diabetes. Crit Care Resusc. 2017;19(2):167–174. Google Scholar | Medline
|
| 47. | Pasquel, FJ, Klonoff, DC. COVID in diabetes. https://www.covidindiabetes.org/. Accessed September 10, 2020. Google Scholar
|
| 48. | Krinsley, J, Schultz, MJ, Spronk, PE, et al. Mild hypoglycemia is strongly associated with increased intensive care unit length of stay. Ann Intensive Care. 2011;1:49. Google Scholar | Crossref | Medline | ISI
|
| 49. | Galindo, RJ, Umpierrez, GE. Management of diabetes and/or hyperglycemia in non-critical care hospital settings. In: Matfin, G (ed.) Endocrine and Metabolic Medical Emergencies. Hoboken, BJ: John Wiley & Sons, Ltd; 2018:491–505. Google Scholar
|
| 50. | Goldberg, PA, Siegel, MD, Russell, RR, et al. Experience with the continuous glucose monitoring system in a medical intensive care unit. Diabetes Technol Ther. 2004;6(3):339–347. Google Scholar | Crossref | Medline
|
| 51. | Vriesendorp, TM, DeVries, JH, Holleman, F, Dzoljic, M, Hoekstra, JBL. The use of two continuous glucose sensors during and after surgery. Diabetes Technol Ther. 2005;7(2):315–322. Google Scholar | Crossref | Medline
|
| 52. | Corstjens, AM, Ligtenberg, JJM, van der Horst, ICC, et al. Accuracy and feasibility of point-of-care and continuous blood glucose analysis in critically ill ICU patients. Crit Care. 2006;10(5):R135. Google Scholar | Crossref | Medline | ISI
|
| 53. | De Block, C, Manuel-Y-Keenoy, B, Van Gaal, L, Rogiers, P. Intensive insulin therapy in the intensive care unit: assessment by continuous glucose monitoring. Diabetes Care. 2006;29(8):1750–1756. Google Scholar | Crossref | Medline | ISI
|
| 54. | Price, GC, Stevenson, K, Walsh, TS. Evaluation of a continuous glucose monitor in an unselected general intensive care population. Crit Care Resusc. 2008;10(3):209–216. Google Scholar | Medline
|
| 55. | Holzinger, U, Warszawska, J, Kitzberger, R, Herkner, H, Metnitz, PGH, Madl, C. Impact of shock requiring norepinephrine on the accuracy and reliability of subcutaneous continuous glucose monitoring. Intensive Care Med. 2009;35(8):1383–1389. Google Scholar | Crossref | Medline | ISI
|
| 56. | Rabiee, A, Andreasik, V, Abu-Hamdah, R, et al. Numerical and clinical accuracy of a continuous glucose monitoring system during intravenous insulin therapy in the surgical and burn intensive care units. J Diabetes Sci Technol. 2009;3(4):951–959. Google Scholar | SAGE Journals
|
| 57. | Yamashita, K, Okabayashi, T, Yokoyama, T, et al. Accuracy and reliability of continuous blood glucose monitor in post-surgical patients. Acta Anaesthesiol Scand. 2009;53(1):66–71. Google Scholar | Crossref | Medline | ISI
|
| 58. | Logtenberg, SJ, Kleefstra, N, Snellen, FT, et al. Pre- and postoperative accuracy and safety of a real-time continuous glucose monitoring system in cardiac surgical patients: a randomized pilot study. Diabetes Technol Ther. 2009;11(1):31–37. Google Scholar | Crossref | Medline | ISI
|
| 59. | Holzinger, U, Warszawska, J, Kitzberger, R, et al. Real-time continuous glucose monitoring in critically ill patients: a prospective randomized trial. Diabetes Care. 2010;33(3):467–472. Google Scholar | Crossref | Medline | ISI
|
| 60. | Jacobs, B, Phan, K, Bertheau, L, Dogbey, G, Schwartz, F, Shubrook, J. Continuous glucose monitoring system in a rural intensive care unit: a pilot study evaluating accuracy and acceptance. J Diabetes Sci Technol. 2010;4(3):636–644. Google Scholar | SAGE Journals
|
| 61. | Brunner, R, Kitzberger, R, Miehsler, W, Herkner, H, Madl, C, Holzinger, U. Accuracy and reliability of a subcutaneous continuous glucose-monitoring system in critically ill patients. Crit Care Med. 2011;39(4):659–664. Google Scholar | Crossref | Medline | ISI
|
| 62. | Lorencio, C, Leal, Y, Bonet, A, et al. Real-time continuous glucose monitoring in an intensive care unit: better accuracy in patients with septic shock. Diabetes Technol Ther. 2012;14(7):568–575. Google Scholar | Crossref | Medline | ISI
|
| 63. | Kalmovich, B, Bar-Dayan, Y, Boaz, M, Wainstein, J. Continuous glucose monitoring in patients undergoing cardiac surgery. Diabetes Technol Ther. 2012;14(3):232–238. Google Scholar | Crossref | Medline | ISI
|
| 64. | Kopecký, P, Mráz, M, Bláha, J, et al. The use of continuous glucose monitoring combined with computer-based eMPC algorithm for tight glucose control in cardiosurgical ICU. BioMed Res Int. 2013;2013:186439. Google Scholar | Crossref | Medline | ISI
|
| 65. | Leelarathna, L, English, SW, Thabit, H, et al. Feasibility of fully automated closed-loop glucose control using continuous subcutaneous glucose measurements in critical illness: a randomized controlled trial. Crit Care. 2013;17(4):R159. Google Scholar | Crossref | Medline
|
| 66. | Rodríguez-Quintanilla, KA, Lavalle-González, FJ, Mancillas-Adame, LG, Zapata-Garrido, AJ, Villarreal-Pérez, JZ, Tamez-Pérez, HE. Continuous glucose monitoring in acute coronary syndrome. Arch Cardiol Mex. 2013;83(4):237–243. Google Scholar | Crossref | Medline
|
| 67. | Schuster, KM, Barre, K, Inzucchi, SE, Udelsman, R, Davis, KA. Continuous glucose monitoring in the surgical intensive care unit: concordance with capillary glucose. J Trauma Acute Care Surg. 2014;76(3):798–803. Google Scholar | Crossref | Medline
|
| 68. | Boom, DT, Sechterberger, MK, Rijkenberg, S, et al. Insulin treatment guided by subcutaneous continuous glucose monitoring compared to frequent point-of-care measurement in critically ill patients: a randomized controlled trial. Crit Care. 2014;18(4):453. Google Scholar | Crossref | Medline | ISI
|
| 69. | Umbrello, M, Salice, V, Spanu, P, et al. Performance assessment of a glucose control protocol in septic patients with an automated intermittent plasma glucose monitoring device. Clin Nutr. 2014;33(5):867–871. Google Scholar | Crossref | Medline
|
| 70. | van Hooijdonk, RTM, Leopold, JH, Winters, T, et al. Point accuracy and reliability of an interstitial continuous glucose-monitoring device in critically ill patients: a prospective study. Crit Care. 2015;19:34. Google Scholar | Crossref | Medline | ISI
|
| 71. | Sechterberger, MK, van der Voort, PHJ, Strasma, PJ, DeVries, JH. Accuracy of intra-arterial and subcutaneous continuous glucose monitoring in postoperative cardiac surgery patients in the ICU. J Diabetes Sci Technol. 2015;9(3):663–667. Google Scholar | SAGE Journals
|
| 72. | Punke, MA, Decker, C, Wodack, K, Reuter, DA, Kluge, S. Continuous glucose monitoring on the ICU using a subcutaneous sensor. Med Klin Intensivmed Notfallmedizin. 2015;110(5):360–363. Google Scholar | Crossref
|
| 73. | De Block, CEM, Gios, J, Verheyen, N, et al. Randomized evaluation of glycemic control in the medical intensive care unit using real-time continuous glucose monitoring (REGIMEN Trial). Diabetes Technol Ther. 2015;17(12):889–898. Google Scholar | Crossref | Medline
|
| 74. | Ballesteros, D, Martínez, Ó, Blancas Gómez-Casero, R, et al. Continuous tissue glucose monitoring correlates with measurement of intermittent capillary glucose in patients with distributive shock. Med Intensiva. 2015;39(7):405–411. Google Scholar | Crossref | Medline
|
| 75. | Nohra, E, Buckman, S, Bochicchio, K, et al. Results of a near continuous glucose monitoring technology in surgical intensive care and trauma. Contemp Clin Trials. 2016;50:1–4. Google Scholar | Crossref | Medline
|
| 76. | Wollersheim, T, Engelhardt, LJ, Pachulla, J, et al. Accuracy, reliability, feasibility and nurse acceptance of a subcutaneous continuous glucose management system in critically ill patients: a prospective clinical trial. Ann Intensive Care. 2016;6(1):70. Google Scholar | Crossref | Medline | ISI
|
| 77. | Gottschalk, A, Welp, HA, Leser, L, Lanckohr, C, Wempe, C, Ellger, B. Continuous glucose monitoring in patients undergoing extracorporeal ventricular assist therapy. PLoS One. 2016;11(3):e0148778. Google Scholar | Crossref | Medline | ISI
|
| 78. | Righy Shinotsuka, C, Brasseur, A, Fagnoul, D, So, T, Vincent, J-L, Preiser, J-C. Manual versus automated monitoring accuracy of glucose II (MANAGE II). Crit Care. 2016;20(1):380. Google Scholar | Crossref | Medline
|
| 79. | Schierenbeck, F, Franco-Cereceda, A, Liska, J. Accuracy of 2 different continuous glucose monitoring systems in patients undergoing cardiac surgery. J Diabetes Sci Technol. 2017;11(1):108–116. Google Scholar | SAGE Journals
|
| 80. | Song, I-K, Lee, J-H, Kang, J-E, Park, Y-H, Kim, H-S, Kim, J-T. Continuous glucose monitoring system in the operating room and intensive care unit: any difference according to measurement sites? J Clin Monit Comput. 2017;31(1):187–194. Google Scholar | Crossref | Medline
|
| 81. | Rijkenberg, S, van Steen, SC, DeVries, JH, van der Voort, PHJ. Accuracy and reliability of a subcutaneous continuous glucose monitoring device in critically ill patients. J Clin Monit Comput. 2017;32(5):953–964. Google Scholar | Crossref | Medline
|
| 82. | Bochicchio, GV, Nasraway, S, Moore, L, Furnary, A, Nohra, E, Bochicchio, K. Results of a multicenter prospective pivotal trial of the first inline continuous glucose monitor in critically ill patients. J Trauma Acute Care Surg. 2017;82(6):1049–1054. Google Scholar | Crossref | Medline
|
| 83. | Nukui, S, Akiyama, H, Soga, K, et al. Risk of hyperglycemia and hypoglycemia in patients with acute ischemic stroke based on continuous glucose monitoring. J Stroke Cerebrovasc Dis. 2019;28(12):104346. Google Scholar | Crossref | Medline
|
| 84. | Bridges, BC, Preissig, CM, Maher, KO, Rigby, MR. Continuous glucose monitors prove highly accurate in critically ill children. Crit Care. 2010;14(5):R176. Google Scholar | Crossref | Medline | ISI
|
| 85. | Steil, GM, Langer, M, Jaeger, K, Alexander, J, Gaies, M, Agus, MSD. Value of continuous glucose monitoring for minimizing severe hypoglycemia during tight glycemic control. Pediatr Crit Care Med. 2011;12(6):643–648. Google Scholar | Crossref | Medline | ISI
|
| 86. | Prabhudesai, S, Kanjani, A, Bhagat, I, Ravikumar, KG, Ramachandran, B. Accuracy of a real-time continuous glucose monitoring system in children with septic shock: a pilot study. Indian J Crit Care Med. 2015;19(11):642–647. Google Scholar | Crossref | Medline
|
| 87. | Kotzapanagiotou, E, Tsotridou, E, Volakli, E, et al. Evaluation of continuous flash glucose monitoring in a pediatric ICU setting. J Clin Monit Comput. 2020;34(4):843–852. Google Scholar | Crossref | Medline
|
| 88. | Sopfe, J, Vigers, T, Pyle, L, Giller, RH, Forlenza, GP. Safety and accuracy of factory-calibrated continuous glucose monitoring in pediatric patients undergoing hematopoietic stem cell transplantation. Diabetes Technol Ther. 2020;22(10):727–733. doi:10.1089/dia.2019.0521. Google Scholar | Crossref | Medline
|
| 89. | Howanitz, PJ, Jones, BA, College of American Pathologists . Comparative analytical costs of central laboratory glucose and bedside glucose testing: a College of American Pathologists Q-Probes study. Arch Pathol Lab Med. 2004;128(7):739–745. Google Scholar | Medline | ISI
|
| 90. | Klonoff, DC, Umpierrez, GE, Rice, MJ. A milestone in point of care capillary blood glucose monitoring of critically Ill hospitalized patients. J Diabetes Sci Technol. 2018;12(6):1095–1100. Google Scholar | SAGE Journals
|
| 91. | Marics, G, Koncz, L, Eitler, K, et al. Effects of pH, lactate, hematocrit and potassium level on the accuracy of continuous glucose monitoring (CGM) in pediatric intensive care unit. Ital J Pediatr. 2015;41:17. Google Scholar | Crossref | Medline
|
| 92. | Scrimgeour, LA, Potz, BA, Sellke, FW, Abid, MR. Continuous glucose monitoring in the cardiac ICU: current use and future directions. Clin Med Res. 2017;6(6):173–176. Google Scholar | Crossref
|
| 93. | Gómez, AM, Umpierrez, GE, Muñoz, OM, et al. Continuous glucose monitoring versus capillary point-of-care testing for inpatient glycemic control in type 2 diabetes patients hospitalized in the general ward and treated with a basal bolus insulin regimen. J Diabetes Sci Technol. 2015;10(2):325–329. Google Scholar | SAGE Journals
|
| 94. | Wang, M, Singh, LG, Spanakis, EK. Advancing the use of CGM devices in a non-ICU Setting. J Diabetes Sci Technol. 2019;13(4):674–681. Google Scholar | SAGE Journals
|
| 95. | FreeStyle Libre Pro. Abbott. 2020. https://www.abbott.com/corpnewsroom/products-safety-info-pages/diabetes-freestyle-librepro.html. Accessed September 10, 2020. Google Scholar
|
| 96. | Galindo, RJ, Migdal, AL, Davis, GM, et al. Comparison of the FreeStyle Libre Pro flash continuous glucose monitoring (CGM) system and point-of-care capillary glucose testing (POC) in hospitalized patients with type 2 diabetes (T2D) treated with basal-bolus insulin regimen. Diabetes Care. July 6, 2020. doi:10.2337/dc19-2073. Google Scholar | Crossref
|
| 97. | Dungan, KM, Han, W, Miele, A, Zeidan, T, Weiland, K. Determinants of the accuracy of continuous glucose monitoring in non-critically ill patients with heart failure or severe hyperglycemia. J Diabetes Sci Technol. 2012;6(4):884–891. Google Scholar | SAGE Journals
|
| 98. | Burt, MG, Roberts, GW, Aguilar-Loza, NR, Stranks, SN. Brief report: comparison of continuous glucose monitoring and finger-prick blood glucose levels in hospitalized patients administered basal-bolus insulin. Diabetes Technol Ther. 2013;15(3):241–245. Google Scholar | Crossref | Medline | ISI
|
| 99. | Schaupp, L, Donsa, K, Neubauer, KM, et al. Taking a closer look—continuous glucose monitoring in non-critically Ill hospitalized patients with type 2 diabetes mellitus under basal-bolus insulin therapy. Diabetes Technol Ther. 2015;17(9):611–618. Google Scholar | Crossref | Medline | ISI
|
| 100. | Spanakis, EK, Levitt, DL, Siddiqui, T, et al. The effect of continuous glucose monitoring in preventing inpatient hypoglycemia in general wards: the glucose telemetry system. J Diabetes Sci Technol. 2018;12(1):20–25. Google Scholar | SAGE Journals
|
| 101. | Singh, LG, Levitt, DL, Satyarengga, M, et al. Continuous glucose monitoring in general wards for prevention of hypoglycemia: results from the glucose telemetry system pilot study. J Diabetes Sci Technol. 2020;14(4):783–790. Google Scholar | SAGE Journals
|
| 102. | Nair, BG, Dellinger, EP, Flum, DR, Rooke, GA, Hirsch, IB. A pilot study of the feasibility and accuracy of inpatient continuous glucose monitoring. Diabetes Care. 2020;43(11):e168–e169. doi:10.2337/dc20-0670. Google Scholar | Crossref | Medline
|
| 103. | Ushigome, E, Yamazaki, M, Hamaguchi, M, et al. Usefulness and safety of remote continuous glucose monitoring for a severe COVID-19 patient with diabetes [published online ahead of print July 15, 2020]. Diabetes Technol Ther. doi:10.1089/dia.2020.0237. Google Scholar | Crossref
|
| 104. | ClinicalTrials.gov. U.S . National Library of Medicine. May 7, 2020. https://www.clinicaltrials.gov/. Accessed September 10, 2020. Google Scholar
|
| 105. | Wallia, A, Umpierrez, GE, Nasraway, SA, Klonoff, DC, PRIDE Investigators . Round table discussion on inpatient use of continuous glucose monitoring at the international hospital diabetes meeting. J Diabetes Sci Technol. 2016;10(5):1174–1181. Google Scholar | SAGE Journals
|
| 106. | van Steen, SCJ, Rijkenberg, S, Limpens, J, van der Voort, PHJ, Hermanides, J, DeVries, JH. The clinical benefits and accuracy of continuous glucose monitoring systems in critically ill patients-a systematic scoping review. Sensors. 2017;17(1). Google Scholar
|
| 107. | Pleus, S, Ulbrich, S, Zschornack, E, Kamann, S, Haug, C, Freckmann, G. Documentation of skin-related issues associated with continuous glucose monitoring use in the scientific literature. Diabetes Technol Ther. 2019;21(10):538–545. Google Scholar | Crossref | Medline
|
| 108. | Kamann, S, Heinemann, L, Oppel, E. Usage of hydrocolloid-based plasters in patients who have developed allergic contact dermatitis to isobornyl acrylate while using continuous glucose monitoring systems. J Diabetes Sci Technol. 2020;14(3):582–585. Google Scholar | SAGE Journals
|
| 109. | Freckmann, G, Buck, S, Waldenmaier, D, et al. Skin reaction report form: development and design of a standardized report form for skin reactions due to medical devices for diabetes management [published online ahead of print March 22, 2020]. J Diabetes Sci Technol. doi:10.1177/1932296820911105. Google Scholar | SAGE Journals
|
| 110. | Parienti, J-J, Mongardon, N, Mégarbane, B, et al. Intravascular complications of central venous catheterization by insertion site. N Engl J Med. 2015;373(13):1220–1229. Google Scholar | Crossref | Medline | ISI
|
| 111. | Bally, L, Thabit, H, Hartnell, S, et al. Closed-loop insulin delivery for glycemic control in noncritical care. N Engl J Med. 2018;379(6):547–556. Google Scholar | Crossref | Medline
|
| 112. | Stewart, ZA, Yamamoto, JM, Wilinska, ME, et al. Adaptability of closed loop during labor, delivery, and postpartum: a secondary analysis of data from two randomized crossover trials in type 1 diabetes pregnancy. Diabetes Technol Ther. 2018;20(7):501–505. Google Scholar | Crossref | Medline
|
| 113. | Bally, L, Gubler, P, Thabit, H, et al. Fully closed-loop insulin delivery improves glucose control of inpatients with type 2 diabetes receiving hemodialysis. Kidney Int. 2019;96(3):593–596. Google Scholar | Crossref | Medline
|
| 114. | Okabayashi, T, Shima, Y, Sumiyoshi, T, et al. Intensive versus intermediate glucose control in surgical intensive care unit patients. Diabetes Care. 2014;37(6):1516–1524. Google Scholar | Crossref | Medline
|
| 115. | Mibu, K, Yatabe, T, Hanazaki, K. Blood glucose control using an artificial pancreas reduces the workload of ICU nurses. J Artif Organs. 2012;15(1):71–76. Google Scholar | Crossref | Medline | ISI
|
| 116. | Thabit, H, Hartnell, S, Allen, JM, et al. Closed-loop insulin delivery in inpatients with type 2 diabetes: a randomised, parallel-group trial. Lancet Diabetes Endocrinol. 2017;5(2):117–124. Google Scholar | Crossref | Medline
|
| 117. | Boughton, CK, Bally, L, Martignoni, F, et al. Fully closed-loop insulin delivery in inpatients receiving nutritional support: a two-centre, open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):368–377. Google Scholar | Crossref | Medline
|
| 118. | Namikawa, T, Munekage, M, Kitagawa, H, et al. Comparison between a novel and conventional artificial pancreas for perioperative glycemic control using a closed-loop system. J Artif Organs. 2017;20(1):84–90. Google Scholar | Crossref | Medline
|
| 119. | Nikkiso Co., Ltd . What is the Artificial Pancreas? http://www.nikkiso.com/products/medical/artificial_pancreas.html. Accessed September 10, 2020. Google Scholar
|
| 120. | Admetsys – Advanced Metabolic Systems . https://admetsys.com/. Accessed September 10, 2020. Google Scholar
|
| 121. | Sardu, C, D’Onofrio, N, Balestrieri, ML, et al. Outcomes in patients with hyperglycemia affected by COVID-19: can we do more on glycemic control? Diabetes Care. 2020;43(7):1408–1415. Google Scholar | Crossref | Medline
|
| 122. | Umpierrez, GE, Klonoff, DC. Diabetes technology update: use of insulin pumps and continuous glucose monitoring in the hospital. Diabetes Care. 2018;41(8):1579–1589. Google Scholar | Crossref | Medline
|
| 123. | Thompson, B, Korytkowski, M, Klonoff, DC, Cook, CB. Consensus statement on use of continuous subcutaneous insulin infusion therapy in the hospital. J Diabetes Sci Technol. 2018;12(4):880–889. Google Scholar | SAGE Journals
|
| 124. | Cook, CB, Beer, KA, Seifert, KM, Boyle, ME, Mackey, PA, Castro, JC. Transitioning insulin pump therapy from the outpatient to the inpatient setting: a review of 6 years’ experience with 253 cases. J Diabetes Sci Technol. 2012;6(5):995–1002. Google Scholar | SAGE Journals
|
| 125. | Noschese, ML, DiNardo, MM, Donihi, AC, et al. Patient outcomes after implementation of a protocol for inpatient insulin pump therapy. Endocr Pract. 2009;15(5):415–424. Google Scholar | Crossref | Medline | ISI
|
| 126. | Thompson, B, Leighton, M, Korytkowski, M, Cook, CB. An overview of safety issues on use of insulin pumps and continuous glucose monitoring systems in the hospital. Curr Diab Rep. 2018;18(10):81. Google Scholar | Crossref | Medline
|
| 127. | Mensh, BD, Wisniewski, NA, Neil, BM, Burnett, DR. Susceptibility of interstitial continuous glucose monitor performance to sleeping position. J Diabetes Sci Technol. 2013;7(4):863–870. Google Scholar | SAGE Journals
|
| 128. | Baysal, N, Cameron, F, Buckingham, BA, et al. A novel method to detect pressure-induced sensor attenuations (PISA) in an artificial pancreas. J Diabetes Sci Technol. 2014;8(6):1091–1096. Google Scholar | SAGE Journals
|
| 129. | Buchanan, J, Zabinsky, J, Ferrara-Cook, C, Adi, S, Wong, J. Comparison of insulin pump bolus calculators reveals wide variation in dose recommendations. J Diabetes Sci Technol. September 2020. E-Pub Ahead of Print. Google Scholar | SAGE Journals
|
| 130. | Thabit, H, Hovorka, R. Bridging technology and clinical practice: innovating inpatient hyperglycaemia management in non-critical care settings. Diabet Med. 2018;35(4):460–471. Google Scholar | Crossref | Medline
|
| 131. | Partridge, H, Perkins, B, Mathieu, S, Nicholls, A, Adeniji, K. Clinical recommendations in the management of the patient with type 1 diabetes on insulin pump therapy in the perioperative period: a primer for the anaesthetist. Br J Anaesth. 2016;116(1):18–26. Google Scholar | Crossref | Medline
|
| 132. | Carayon, P, Hundt, AS, Karsh, B, et al. Work system design for patient safety: the SEIPS model. Qual Saf Health Care. 2006;15(suppl 1):i50–i58. Google Scholar | Crossref | Medline
|
| 133. | Heinemann, L, Klonoff, DC. An opportunity to increase the benefit of CGM usage: the need to train the patients adequately. J Diabetes Sci Technol. 2020;14(6):983–986. doi:10.1177/1932296819895083. Google Scholar | SAGE Journals
|
| 134. | Freckmann, G, Pleus, S, Grady, M, Setford, S, Levy, B. Measures of accuracy for continuous glucose monitoring and blood glucose monitoring devices. J Diabetes Sci Technol. 2019;13(3):575–583. Google Scholar | SAGE Journals
|
| 135. | McClellan, M, Gadina, S, Daniel, G, Deak, D, Bryan, J, Streit, C. Unique Device Identifiers (UDIs): A Roadmap for Effective Implementation. The Brookings Institution; 2014. https://www.brookings.edu/wp-content/uploads/2016/06/UDI-Final-12052014-1.pdf. Accessed September 10, 2020. Google Scholar
|
| 136. | Center for Devices and Radiological Health. Unique device identification: policy regarding compliance dates for class i and unclassified devices and certain devices requiring direct marking . U.S. Food and Drug Administration. June 29, 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/unique-device-identification-policy-regarding-compliance-dates-class-i-and-unclassified-devices-and. Accessed September 10, 2020. Google Scholar
|
| 137. | Henry, J, Pylypchuk, Y, Searcy, T, Patel, V. Adoption of electronic health record systems among U.S. non-federal acute care hospitals: 2008-2015. The Office of the National Coordinator for Health Information Technology. May 2016. https://dashboard.healthit.gov/evaluations/data-briefs/non-federal-acute-care-hospital-ehr-adoption-2008-2015.php. Accessed September 10, 2020. Google Scholar
|
| 138. | Kumar, RB, Goren, ND, Stark, DE, Wall, DP, Longhurst, CA. Automated integration of continuous glucose monitor data in the electronic health record using consumer technology. J Am Med Inform Assoc. 2016;23(3):532–537. Google Scholar | Crossref | Medline
|
| 139. | Wong, JC, Izadi, Z, Schroeder, S, et al. A pilot study of use of a software platform for the collection, integration, and visualization of diabetes device data by health care providers in a multidisciplinary pediatric setting. Diabetes Technol Ther. 2018;20(12):806–816. Google Scholar | Crossref | Medline
|
| 140. | Espinoza, J, Shah, P, Raymond, J. Integrating continuous glucose monitor data directly into the electronic health record: proof of concept. Diabetes Technol Ther. 2020;22(8):570–576. doi:10.1089/dia.2019.0377. Google Scholar | Crossref | Medline
|
| 141. | Schulz, S, Stegwee, R, Chronaki, C. Standards in healthcare data. In: Kubben, P, Dumontier, M, Dekker, A (eds) Fundamentals of Clinical Data Science. Springer, Cham; 2019:19–36. Google Scholar
|
| 142. | U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S . Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4(1):79. doi:10.1186/1477-7525-4-79. Google Scholar | Crossref | Medline
|
| 143. | Marrero, DG, Hilliard, ME, Maahs, DM, McAuliffe-Fogarty, AH, Hunter, CM. Using patient reported outcomes in diabetes research and practice: Recommendations from a national workshop. Diabetes Res Clin Pract. 2019;153:23–29. Google Scholar | Crossref | Medline
|
| 144. | Mathioudakis, N, Pronovost, PJ, Cosgrove, SE, Hager, D, Golden, SH. Modeling inpatient glucose management programs on hospital infection control programs: an infrastructural model of excellence. Jt Comm J Qual Patient Saf. 2015;41(7):325–336. Google Scholar | Crossref | Medline
|
| 145. | Cardona, S, Pasquel, FJ, Fayfman, M, et al. Hospitalization costs and clinical outcomes in CABG patients treated with intensive insulin therapy. J Diabetes Complications. 2017;31(4):742–747. Google Scholar | Crossref | Medline
|
| 146. | Cruz, P, Blackburn, MC, Tobin, GS. A systematic approach for the prevention and reduction of hypoglycemia in hospitalized patients. Curr Diab Rep. 2017;17(11):11. Google Scholar | Crossref | Medline
|
| 147. | State Operations Manual Appendix C – Survey procedures and interpretive guidelines for laboratories and laboratory services . 2019. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/som107ap_c_lab.pdf. Accessed September 10, 2020. Google Scholar
|
| 148. | Revision of the “guideline of the German medical association on quality assurance in medical laboratory examinations – Rili-BAEK” (unauthorized translation) . J Lab Med. 2015;39(1):26–69. Google Scholar
|
| 149. | United States Supreme Court . Official Reports of the Supreme Court. Vol 531 Part 2. (Wagner FD, ed.). Supreme Court; 2001. Google Scholar
|
| 150. | Center for Devices and Radiological Health. Software as a medical device (SAMD): clinical evaluation. U.S . Food and Drug Administration. March 2, 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/software-medical-device-samd-clinical-evaluation. Accessed September 10, 2020. Google Scholar
|
| 151. | Klonoff, DC. Cybersecurity for connected diabetes devices. J Diabetes Sci Technol. 2015;9(5):1143–1147. Google Scholar | SAGE Journals
|
| 152. | Carvalho, JV, Rocha, Á, Abreu, A. Maturity assessment methodology for HISMM – hospital information system maturity model. J Med Syst. 2019;43(2):35. Google Scholar | Crossref | Medline
|
| 153. | Carvalho, JV, Rocha, Á, Abreu, A. Maturity models of healthcare information systems and technologies: a literature review. J Med Syst. 2016;40(6):131. Google Scholar | Crossref | Medline |
Author Affiliations:
ECO #0958-A