Publication
Journal of Diabetes Science and Technology
Date
June 23, 2021
Authors
David C. Klonoff, MD, FACP, FRCP (Edin), Fellow AIMBE1, Jordan Messler, MD, SFHM, FACP2, Timothy Valk, MD, FACP, FACE3, Ram Jagannathan, Ph.D.4, Francisco J. Pasquel, MD, MPH4, Guillermo E. Umpierrez, MD, CDE4
Corresponding Author:
David C. Klonoff, M.D., FACP, FRCP (Edin), Fellow AIMBE, Medical Director, Diabetes Research Institute, Mills-Peninsula Medical Center, 100 South San Mateo Drive, Room 5147, San Mateo, CA 94401, USA. Email: dklonoff@diabetestechnology.org
Abstract
Complications of Coronavirus Disease 2019 (COVID-19) occur with increased frequency in people admitted to the hospital with diabetes or hyperglycemia. The increased risk for COVID-19 infections in the presence of these metabolic conditions is in part due to overlapping pathophysiologic features of COVID-19, diabetes, and glucose control. Various antiviral treatments are being tested in COVID-19 patients. We believe that in these trials, it will be useful to evaluate treatment effect differences in patients stratified according to whether they have diabetes or hyperglycemia. In this way, it will be possible to better facilitate development of antiviral treatments that are most specifically beneficial for the large subset of COVID-19 patients who have diabetes or hyperglycemia.
Keywords
diabetes, COVID-19, hospital, clinical trial, antiviral, hyperglycemia
Introduction
Hyperglycemia in hospitalized Coronavirus Disease 2019 (COVID-19) patients is common and leads to worse outcomes. Despite this, hyperglycemia is infrequently measured as a factor affecting outcomes in clinical trials of COVID-19 treatments. In this two-part commentary, in part one we discuss three topics related to the intersection of these two diseases: (1) mechanisms of how the pathophysiology of both COVID-19 and diabetes overlap and lead to to worse outcomes than for patients without diabetes, (2) the assessment of glycemia in current research on treatments for COVID-19, and (3) a proposal that diabetes and glycemia be considered significant risk factors worthy of being stratified for outcomes of clinical trials of treatments for COVID-19. This proposed approach to antiviral treatments will be useful for managing diabetes patients with COVID-19 and potentially future pandemic viral infections as well.
Diabetes and Hyperglycemia are Risk Factors for Adverse Outcomes in COVID-19
Diabetes and hyperglycemia are common in hospitalized patients with COVID-19. The hospital mortality of COVID-19 with diabetes and stress-induced hyperglycemia is approximately 30% and 40%, respectively.1,2 The prevalence of COVID-19 patients with diabetes is not different from the prevalence of diabetes in the general population.3 However, when a person with diabetes develops COVID-19, their risk of severe morbidity is approximately three times higher than in the general population.4 From a series of 1122 COVID-19 patients in 88 U.S. hospitals, we reported in 2020 that the mortality rate was 28.8% for diabetes and/or uncontrolled hyperglycemia patients, compared with 6.2% for patients without diabetes or hyperglycemia.5 We also reported that stress hyperglycemia (defined as being present when two or more BGs > 180 mg/dL occurred within any 24-hour period with an A1C < 6.5% or no A1C testing during hospitalization), compared to known diabetes, has ~threefold higher mortality (41.7% compared to 14.8%).5 These findings for hospitalized COVID-19 patients represented a fourfold increase in mortality with diabetes and a sevenfold increase with stress-induced hyperglycemia compared to those with neither.5 A pair of 2021 meta-analyses on the effects of hyperglycemia on complications of COVID-19 both concluded that, compared to normoglycemic patients, hyperglycemic patients had a higher risk of mortality and poor outcomes.6,7
Relationship Between Hyperglycemia and COVID-19 Morbidity and Mortality
What is the reason for the increased mortality of COVID-19 in the presence of diabetes? Is the problem caused by hyperglycemia and attendant systems breakdown from glucotoxicity, or are the elevated glucose levels a manifestation or epiphenomenon of being severely ill with COVID-19 and an effect rather than a cause of the increased risk of mortality?
A recent multicenter, retrospective hospital-based analysis of 1544 diabetes patients with COVID-19 from 91 hospitals in 12 states in the United States examined this question. An association was sought between achieving inpatient glycemic control and clinical outcomes in hospitalized patients with COVID-19.8 This relationship was assessed in a context of whether achieved glycemia within a window where traditional glycemic goals can be met (2–3 days for non-ICU patients or within 24 hours for those directly admitted to the ICU) would better predict outcomes than admission glycemia. The study found that: (1) the glycemia value at 2–3 days predicted mortality better than baseline values for non-ICU admissions but not for direct ICU admissions, and (2) reaching a mean glucose level of 140-180 mg/dl was associated with lower mortality than higher levels of glycemia (>250 mg/dl). This analysis did not account for the heterogeneity of treatment regimens, which may have been different between ICU settings and across various health systems Although this study demonstrated that treatment to achieve glycemic goals should be effective in improving morbidity and mortality, the analysis could not discern which specific COVID-19 antiviral treatments were more or less likely to be effective in the presence of stress-induced hyperglycemia.
Mechanisms of Morbidity Due to COVID-19
Multiple mechanisms have been hypothesized to be responsible for the morbidity and mortality of COVID-19. Each is a potential target for treatments or vaccinations. In each case, hyperglycemia may exacerbate the mechanism to increase the risk of poor outcomes. Seven mechanisms that can influence the infectivity of the SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) virus are listed in Table 1.
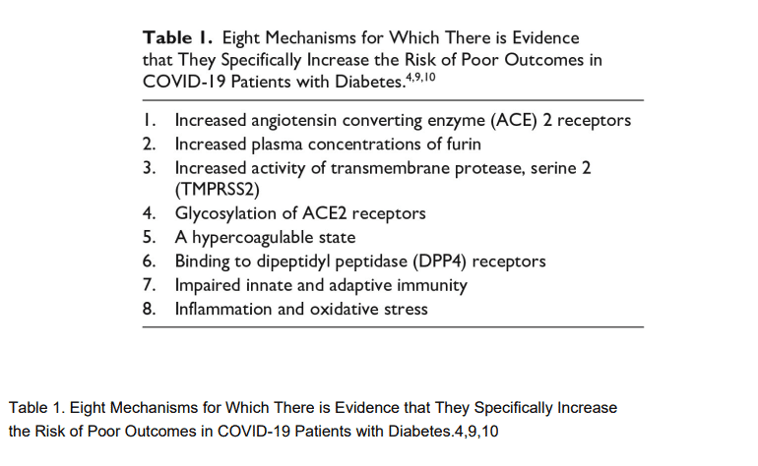
Multiple underlying mechanisms explain the poor outcome of COVID-19 in patients with hyperglycemia and diabetes. SARS-CoV-2 uses ACE2 receptors to enter affected cells.11 Diabetes can increase the expression of ACE2, which could increase susceptibility to infection by this virus.12 The cell membrane-bound enzyme furin cleaves the COVID-19 spike protein at a cleavage site between its S1 and S2 subunits, which is a process that is required for viral entry into cells.13 Following cleavage, fusion of the spike protein is activated by a second membrane-bound enzyme, TMPRSS2, that cleaves a site on the S2 subunit called the S2’ site.14 Plasma levels of shed furin in the circulation correlate with blood glucose levels and predict type 2 diabetes development.15 Little is known about whether TMPRSS2 activity is affected by diabetes, but increased circulating plasma concentrations of the serine protease enzyme, granzyme B, have been found in type 2 diabetes.16 Glycosylation of the ACE2 receptor and the SARS-CoV-2 spike protein increases the binding of the SARS-CoV-2 to that receptor.17 The hyperglycemia of diabetes might increase the glycosylation of ACE2 receptors18 or of the SARS-CoV-2 spike protein, which utilizes a glycan shield to thwart the host immune response and contribute to increased binding of this virus19 and lead to worse outcomes.20 SARS-CoV-2 can cause inflammation of vascular endothelial cells, which can, in turn, activate coagulation pathways, causing the formation of microthrombi in blood vessels. Type 2 diabetes is also associated with endothelial dysfunction and platelet aggregation, which can also cause a hypercoagulable state.21 A high affinity between human dipeptidyl peptidase 4 (DPP4), a cell-surface protease that cleaves dipeptides, and the spike protein of SARS-CoV-2 has been reported, which has led to this enzyme to be considered as a candidate binding target for the spike protein.22 There is limited evidence of increased circulating levels of soluble DPP4 receptors in diabetes,23 as well as little evidence, but no strong evidence to date, that this receptor is a significant target of the SARS-CoV-2 virus.24,25 Innate immunity is an immediate first line non-specific reponse to pathogens by natural killer cells, macrophages, granulcytes and dendridic cells. Adaptive immunity is a delayed secondline specific reponse to pathogens characterized by clonal expansion of lymphocytes. In both COVID-1926 and type 2 diabetes27 innate and adaptive immunity are impaired and lymphocytes and monocytes are decreased in number and function.28–30 Patients with COVID-19 infections have an exacerbated inflammatory response with increased serum levels of inflammatory markers, such as various cytokines, C-reactive protein, lactic dehydrogenase, ferritin, and D-dimer, all of which may result in cytokine storm.31,32 Hyperglycemia in patients with diabetes leads to an inflammatory state characterized by increased oxidative stress markers33 as well as increased macrophage production of pro-inflammatory cytokines,34 such as tumor necrosis factor-alpha (TNF α), interleukin (IL)-6 and IL-1β, and C-reactive protein.35 These inflammatory substances, in turn, lead to impaired insulin secretion as well as reduced insulin sensitivity.36 Furthermore, the increased inflammatory substances and reactive oxygen species can lead to capillary perturbation and cellular damage of lipids, membranes, proteins, and DNA.37
Treatments Directed at Mechanisms of Morbidity for COVID-19
Many treatments that modify or block molecular mechanisms that promote SARS-CoV-2 infectivity (e.g., antivirals, anti-inflammatories, monoclonal antibodies, anticoagulants, and DPP4 Inhibitors) might be expected to affect people with diabetes differently than people without diabetes. This is because the major factors potentiating SARS-CoV-2 infectivity are generally made worse by hyperglycemia and a diagnosis of diabetes. Likewise, many treatments for hyperglycemia and diabetes might be expected to favorably affect outcomes of COVID-19 infections because hyperglycemia potentiates many of the molecular factors that promote SARS-CoV-2 infectivity.
Treatment Bias
Trials of COVID-19 therapies have not accounted for subgroups of patients with and without diabetes or stress-hyperglycemia. It would be essential to know if there are differential treatment effects according to this important underlying patient characteristic. For example, including the largest clinical trial to date, the RECOVERY trial, there have been no reports on outcomes according to glycemic control (although dexamethasone is known to worsen glycemia). A meta-analysis of seven COVID-19 randomized controlled trials (RCTs) reported that corticosteroid use was associated with lower all-cause mortality at 28 days after randomization.38 There was a subgroup analysis including important variables such as age, gender, and sex, but there is no mention of the effect of steroids on glycemic control in this population, and the potential differential effect.
Given the possible impact of various treatments on: (a) glucose metabolism, (b) the immune response in a physiological background affected by glucose or long-standing diabetes, or (c) the interaction with medications for diabetes that could modify the response to therapy or severity of illness, we recommend at least defining a-priori subgroup analyses that account for these important covariates in undergoing or future clinical trials.
Trials of COVID-19 therapies have not adjusted for glycemia, which could have accounted for different outcomes in patients with/without diabetes and stress hyperglycemia. Diabetes and glycemic control are important confounders for outcomes in COVID-19, which have not been commonly accounted for in RCTs of therapies for COVID-19. Given the pervasiveness of hyperglycemia and diabetes and the lack of ability to control hyperglycemia well in the hospital, controlling for hyper and hypoglycemia would be an essential aspect of studies.
In the hospital trials, blood glucose data is stored in the electronic records on all patients. That data can be analyzed even now and correlated with outcomes. We believe that a worthy research project would be to obtain and combine data from a large set of trials of COVID-19 therapies and proceed with a secondary analysis of outcomes stratified according to the presence of diabetes and hyperglycemia. The study would assess whether patients with diabetes had better outcomes or not and what was the impact of these interventions for COVID-19 on glycemic control.
Trials of Therapies for COVID-19
We present summaries of nine major clinical trials of antiviral therapy for COVID-19 (see Table 2).39–46 In July 2020, The RECOVERY Trial reported benefits of a 10 day course of dexamethasone in patients requiring oxygen.39 Steroids have been shown to cause hyperglycemia in greater than 50% of patients without diabetes and more than 80% of patients with diabetes.47 In patients with chronic obstructive pulmonary disease, one study showed that for every 18 mg/dl increase in glucose was an associated 15% increase in adverse events.48 In patients with sepsis, the 2008 CORTICUS trial concluded that the hyperglycemia related to steroids might have impacted mortality.49 Hypoglycemia is an important variable as well, as patients with sepsis, hypoglycemia, and no diabetes have a higher mortality.50
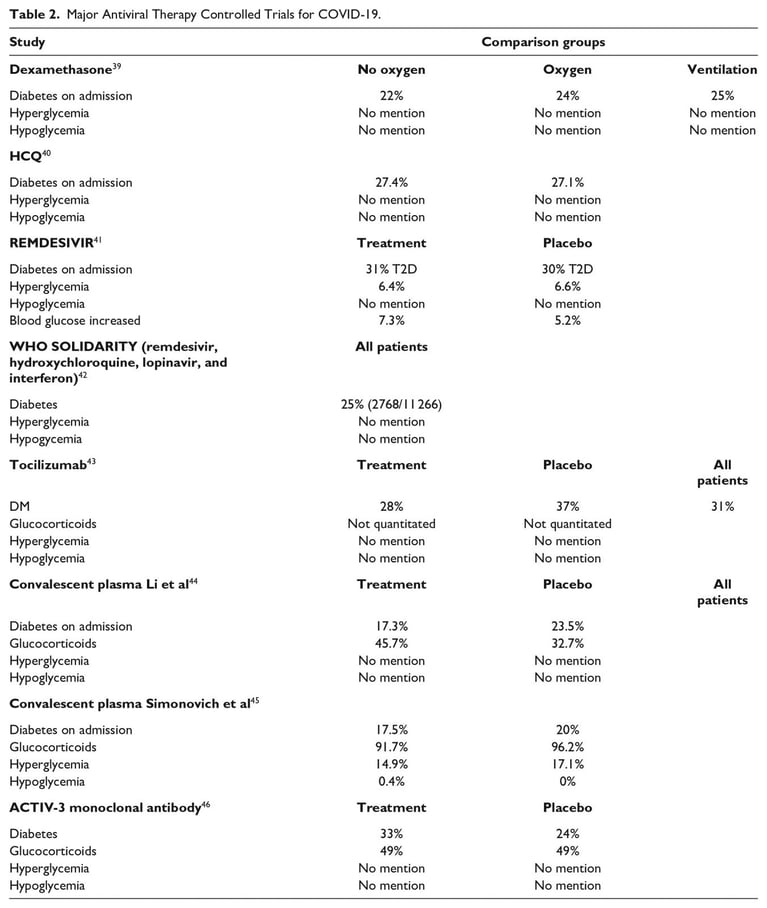
Given the pervasiveness of hyperglycemia in the hospital,51,52 glycemic control should be ensured and reported. Additionally, is it possible that controlling for hyperglycemia and hypoglycemia could impact the results of COVID-19 inpatient treatment studies? Perhaps even more favorably? These same comments can be made regarding any trial examining the impact of steroids, COVID-19 or not.
Hydroxychloroquine (HCQ) was studied extensively early in the pandemic, with RCTs showing no benefit for the use of HCQ in the hospital. Patients on HCQ had a higher in hospital mortality and were more likely to be ventilated.40 No record of hypoglycemia or hyperglycemia rates are mentioned in the study, though HCQ can impact glycemia by increasing the risk for hypoglycemia, and in longer-term studies, reducing the rate of diabetes incidence.53
The ACCT-1 trial evaluated remdesivir in hospitalized patients with COVID-19, showing an improved recovery time but no mortality benefit.41 Diabetes at baseline was reported, and glucose levels were evaluated as an endpoint in the treatment protocol, as a safety measure, but hyperglycemia and hypoglycemia rates were not accounted for as confounders. Elevated blood glucose and hyperglycemia were separately noted, with no definitions reported, and a combined rate of 13.7% in the treatment arm and 11.8% in the placebo group. In this study, the data in the article’s Supplement Table 20 and the data in the article’s Supplement Table 1 show a significant number with diabetes (31% in treatment group) compared to a significantly lower number with hyperglycemia (13.7%).41 This incongruent finding leaves doubts in the assessment of diabetes and glycemia.
The SOLIDARITY Trial reported on 2750 patients receiving remdesivir, 954 patients receiving HCQ, 1411 lopinavir patients, and 2063 patients receiving interferon, with outcomes showing minimal to no impact on mortality, length of stay, or ventilator requirement.42 Baseline diabetes and corticosteroid use are reported, with no mention of glycemic outcomes.
Tocilizumab, an interleukin-6 (IL-6) receptor monoclonal antibody, was examined in an RCT of patients with severe SARS-CoV-2, in 243 patients, showing no impact on preventing mechanical ventilation or death.43 In the Stone article there was a greater percentage of diabetic patients in the placebo group (37% vs 28%) which could skew the data. Diabetes and glucocorticoid use was reported at baseline. However, no glycemic outcomes were reported, though inhibiting IL-6 can impact glycemia, by improving insulin sensitivity.54 In addition, patients with COVID-19, with hyperglycemia, have higher levels of IL-6, and a small analysis suggested that higher levels of IL-6 on admission minimized the impact of IL-6 inhibitors.55
Convalescent studies have not shown clear benefits, though they continue to be used as treatments. An early trial of 103 patients44 reported no clear benefit. In this trial, aside from diabetes status on admission, there is no mention of glycemic outcomes. A more recent trial45 of severe COVID-19 pneumonia patients randomized to placebo or convalescent plasma also showed no clear benefit of plasma. They report hyperglycemia rates of less than 20%, though more than 90% were on steroids for treatment, and approximately 20% had diabetes.
An early 2021 randomized controlled trial of a neutralizing monoclonal antibody, with all patients receiving remdesivir and 49% receiving glucocorticoids, showed no benefit in hospitalized patients with COVID-19.46 Diabetes was reported in 33% of treatment patients and 24% of placebo patients, though no mention of hyperglycemia outcomes are reported.
Trials have evaluated the impact of full dose and therapeutic doses of anticoagulation. Many of them were halted because of worse outcomes with anticoagulants. Given the interaction of thrombosis and insulin resistance, and the more than twofold increased risk for venous thromboembolism development in patients with diabetes,56 adjusting for diabetes and glycemia in these studies will be imperative as well.
Conclusions
Studies of treatments for COVID-19 have generally not considered glycemic control, diabetes status, or admission hyperglycemia when assessing the impact of the studied treatment. The high frequency of diabetes and its influence on outcome and mortality in hospitalized COVID-19 patients mandates a better understanding of the effectiveness of treatments specific for COVID-19 in patients with diabetes and newly diagnosed (stress) hyperglycemia.57 Therapeutic trials for COVID-19 should evaluate the impact of novel interventions on glycemic control and the potential interaction of these interventions with diabetes/hyperglycemic status on treatment effect. Without accounting for glucose control in clinical studies of viral diseases, the effect of therapeutic antiviral agents will be questionable.
In conclusion, we believe that stratified data from large clinical trials may help identify and individualize treatment options for the vulnerable diabetes population. This approach to seeking antiviral treatments that provide optimal outcomes for diabetes patients will be useful for COVID-19 and will probably be useful in the event of any future pandemic infections.
Acknowledgments
The authors would like to acknowledge Annamarie Sucher-Jones for her editorial expertise.
Abbreviations
ACE, angiotensin converting enzyme; COVID-19, coronavirus disease 2019; DPP4, dipeptdyl peptidase-4; HCQ, hydroxychloroquine; RCTs, randomized controlled trials; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TMPRSS2, transmembrane protease, serine 2; TNF α, tumor necrosis factor-alpha.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DCK is a consultant for EoFlow, Fractyl, Lifecare, Novo, Roche Diagnostics, Samsung, and Thirdwayv. JM is an employee of Glytec. TV is a full time employee of Admetsys Corporation. RJ has nothing to disclose. FJP has received research support from Dexcom and Merck and consulting fees from Boehringer Ingelheim, outside the submitted work. He is partially supported by the National Institutes of Health under award numbers1K23GM128221-01A3 and P30DK111024-05S1. GEU is partly supported by research grants from the NIH/NATS UL1 TR002378 and 1P30DK111024-05, and P30DK111024-05S and has received unrestricted research support (to Emory University) from Astra Zeneca, Novo Nordisk, and Dexcom.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article
References
1. Huang, C, Soleimani, J, Herasevich, S, et al. Clinical Characteristics, Treatment, and outcomes of critically ill patients with COVID-19: a scoping review. Mayo Clin Proc. 2021;96(1):183-202. doi:10.1016/j.mayocp.2020.10.022
2. Singh, AK, Singh, R. Hyperglycemia without diabetes and new-onset diabetes are both associated with poorer outcomes in COVID-19. Diabetes Res Clin Pract. 2020;167:108382. doi:10.1016/j.diabres.2020.108382
3. Iughetti, L, Trevisani, V, Cattini, U, et al. COVID-19 and type 1 diabetes: concerns and challenges. Acta Biomed. 2020;91(3):e2020033. doi:10.23750/abm.v91i3.10366
4. Abu-Farha, M, Al-Mulla, F, Thanaraj, TA, et al. Impact of diabetes in patients diagnosed with COVID-19. Front Immunol. 2020;11:576818. doi:10.3389/fimmu.2020.576818
5. Bode, B, Garrett, V, Messler, J, et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14(4):813-821. doi:10.1177/1932296820924469
6. Lazarus, G, Audrey, J, Wangsaputra, VK, Tamara, A, Tahapary, DL. High admission blood glucose independently predicts poor prognosis in COVID-19 patients: a systematic review and dose-response meta-analysis. Diabetes Res Clin Pract. 2021;171:108561.
7. Yang, Y, Cai, Z, Zhang, J. Hyperglycemia at admission is a strong predictor of mortality and severe/critical complications in COVID-19 patients: a meta-analysis. Biosci Rep. 2021;41(2): BSR20203584. doi:10.1042/BSR20203584.
8. Klonoff, DC, Messler, JC, Umpierrez, GE, et al. Association between achieving inpatient glycemic control and clinical outcomes in hospitalized patients with COVID-19: a multicenter, retrospective hospital-based analysis. Diabetes Care. 2021;44(2)578-585.
9. Liao, YH, Zheng, JQ, Zheng, CM, Lu, KC, Chao, YC. Novel molecular evidence related to COVID-19 in patients with diabetes mellitus. J Clin Med. 2020;9(12):3962. doi:10.3390/jcm9123962.
10. Rajpal, A, Rahimi, L, Ismail-Beigi, F. Factors leading to high morbidity and mortality of COVID-19 in patients with type 2 diabetes. J Diabetes. 2020;12(12):895-908. doi:10.1111/1753-0407.13085
11. Bourgonje, AR, Abdulle, AE, Timens, W, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020;251(3):228-248. doi:10.1002/path.5471
12. Rao, S, Lau, A, So, HC. Exploring diseases/traits and blood proteins causally related to expression of ACE2, the putative receptor of SARS-CoV-2: a mendelian randomization analysis highlights tentative relevance of diabetes-related traits. Diabetes Care. 2020;43(7):1416-1426. doi:10.2337/dc20-0643
13. Wu, Y, Zhao, S. Furin cleavage sites naturally occur in coronaviruses. Stem Cell Res. 2020;50:102115. doi:10.1016/j.scr.2020.102115
14. Bestle, D, Heindl, MR, Limburg, H, et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci Alliance. 2020;3(9):e202000786. doi:10.26508/lsa.202000786
15. Fernandez, C, Rysa, J, Almgren, P, et al. Plasma levels of the proprotein convertase furin and incidence of diabetes and mortality. J Intern Med. 2018;284(4):377-387. doi:10.1111/joim.12783.
16. El Mesallamy, HO, Hamdy, NM, Mostafa, DM, Amin, AI. The serine protease granzyme B as an inflammatory marker, in relation to the insulin receptor cleavage in human obesity and type 2 diabetes mellitus. J Interferon Cytokine Res. 2014;34(3):179-186. doi:10.1089/jir.2013.0059.
17. Zhao, P, Praissman, JL, Grant, OC, et al. Virus-receptor interactions of glycosylated SARS-CoV-2 spike and human ACE2 receptor. Cell Host Microbe. 2020;28(4):586-601 e6. doi:10.1016/j.chom.2020.08.004
18. Bagdonaite, I, Wandall, HH. Global aspects of viral glycosylation. Glycobiology. 2018;28(7):443-467. doi:10.1093/glycob/cwy021
19. Casalino, L, Gaieb, Z, Goldsmith, JA, et al. Beyond shielding: the roles of glycans in the SARS-CoV-2 spike protein. ACS Cent Sci. 2020;6(10):1722-1734. doi:10.1021/acscentsci.0c01056
20. Sartore, G, Ragazzi, E, Faccin, L, Lapolla, A. A role of glycation and methylation for SARS-CoV-2 infection in diabetes? Med Hypotheses. 2020;144:110247. doi:10.1016/j.mehy.2020.110247
21. Apicella, M, Campopiano, MC, Mantuano, M, Mazoni, L, Coppelli, A, Del Prato, S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8(9):782-792. doi:10.1016/S2213-8587(20)30238-2
22. Li, Y, Zhang, Z, Yang, L, et al. The MERS-CoV receptor DPP4 as a candidate binding target of the SARS-CoV-2 spike. iScience. 2020;23(8):101400. doi:10.1016/j.isci.2020.101400
23. Ryskjaer, J, Deacon, CF, Carr, RD, et al. Plasma dipeptidyl peptidase-IV activity in patients with type-2 diabetes mellitus correlates positively with HbAlc levels, but is not acutely affected by food intake. Eur J Endocrinol. 2006;155(3):485-493. doi:10.1530/eje.1.02221
24. Solerte, SB, D’Addio, F, Trevisan, R, et al. Sitagliptin treatment at the time of hospitalization was associated with reduced mortality in patients with type 2 diabetes and COVID-19: a multicenter, case-control, retrospective, observational study. Diabetes Care. 2020;43(12):2999-3006. doi:10.2337/dc20-1521
25. Mirani, M, Favacchio, G, Carrone, F, et al. Impact of comorbidities and glycemia at admission and dipeptidyl peptidase 4 inhibitors in patients with type 2 diabetes with COVID-19: a case series from an academic hospital in Lombardy, Italy. Diabetes Care. 2020;43(12):3042-3049. doi:10.2337/dc20-1340
26. Zhou, T, Hu, Z, Yang, S, Sun, L, Yu, Z, Wang, G. Role of adaptive and innate immunity in type 2 diabetes mellitus. J Diabetes Res. 2018;2018:7457269. doi:10.1155/2018/7457269
27. Hue, S, Beldi-Ferchiou, A, Bendib, I, et al. Uncontrolled innate and impaired adaptive immune responses in patients with COVID-19 acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;202(11):1509-1519. doi:10.1164/rccm.202005-1885OC
28. Komura, T, Sakai, Y, Honda, M, Takamura, T, Matsushima, K, Kaneko, S. CD14+ monocytes are vulnerable and functionally impaired under endoplasmic reticulum stress in patients with type 2 diabetes. Diabetes. 2010;59(3):634-643. doi:10.2337/db09-0659.
29. Alzaid, F, Julla, JB, Diedisheim, M, et al. Monocytopenia, monocyte morphological anomalies and hyperinflammation characterise severe COVID-19 in type 2 diabetes. EMBO Mol Med. 2020;12(10):e13038. doi:10.15252/emmm.202013038
30. Kazancioglu, S, Yilmaz, FM, Bastug, A, et al. Lymphocyte subset alteration and monocyte CD4 expression reduction in patients with severe COVID-19. Viral Immunol. Published online November 23, 2020. doi:10.1089/vim.2020.0166
31. Garcia, LF. Immune response, inflammation, and the clinical spectrum of COVID-19. Front Immunol. 2020;11:1441. doi:10.3389/fimmu.2020.01441
32. Henry, BM, de Oliveira, MHS, Benoit, S, Plebani, M, Lippi, G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58(7):1021-1028. doi:10.1515/cclm-2020-0369
33. Burgos-Moron, E, Abad-Jimenez, Z, Maranon, AM, et al. Relationship between oxidative stress, ER stress, and inflammation in type 2 diabetes: the battle continues. J Clin Med. 2019;8(9):1385. doi:10.3390/jcm8091385
34. Chaudhuri, A, Umpierrez, GE. Oxidative stress and inflammation in hyperglycemic crises and resolution with insulin: implications for the acute and chronic complications of hyperglycemia. J Diabetes Complications. 2012;26(4):257-258. doi:10.1016/j.jdiacomp.2012.04.016
35. Morey, M, O’Gaora, P, Pandit, A, Helary, C. Hyperglycemia acts in synergy with hypoxia to maintain the pro-inflammatory phenotype of macrophages. PLoS One. 2019;14(8):e0220577. doi:10.1371/journal.pone.0220577
36. Cieslak, M, Wojtczak, A, Cieslak, M. Role of pro-inflammatory cytokines of pancreatic islets and prospects of elaboration of new methods for the diabetes treatment. Acta Biochim Pol. 2015;62(1):15-21. doi:10.18388/abp.2014_853
37. Fayfman, M, Pasquel, FJ, Umpierrez, GE. Management of hyperglycemic crises: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Med Clin North Am. 2017;101(3):587-606. doi:10.1016/j.mcna.2016.12.011
38. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group ; Sterne, JAC, Murthy, S, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330-1341. doi:10.1001/jama.2020.17023
39. RECOVERY Collaborative Group , Horby, P, Lim, WS, Emberson, JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693-704. doi:10.1056/NEJMoa2021436
40. RECOVERY Collaborative Group , Horby, P, Mafham, M, Linsell, L, et al. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383(21):2030-2040. doi:10.1056/NEJMoa2022926
41. Beigel, JH, Tomashek, KM, Dodd, LE, et al. Remdesivir for the treatment of Covid-19-final report. N Engl J Med. 2020;383(19):1813-1826. doi:10.1056/NEJMoa2007764
42. WHO Solidarity Trial Consortium ; Pan, H, Peto, R, et al. Repurposed antiviral drugs for Covid-19-interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497-511. doi:10.1056/NEJMoa2023184
43. Stone, JH, Frigault, MJ, Serling-Boyd, NJ, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333-2344. doi:10.1056/NEJMoa2028836
44. Li, L, Zhang, W, Hu, Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324(5):460-470. doi:10.1001/jama.2020.10044
45. Simonovich, VA, Burgos Pratx, LD, Scibona, P, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021;384(7):619-629. doi:10.1056/NEJMoa2031304
46. ACTIV-3/TICO LY-CoV555 Study Group , Lundgren, JD, Grund, B, Barkauskas, CE, et al. A neutralizing monoclonal antibody for hospitalized patients with Covid-19. N Engl J Med. 2021;384(10):905-914. doi:10.1056/NEJMoa2033130
47. Clore, JN, Thurby-Hay, L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15(5):469-474. doi:10.4158/EP08331.RAR
48. Baker, EH, Janaway, CH, Philips, BJ, et al. Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease. Thorax. 2006;61(4):284-289. doi:10.1136/thx.2005.051029
49. Sprung, CL, Annane, D, Keh, D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358(2):111-124. doi:10.1056/NEJMoa071366
50. Kushimoto, S, Abe, T, Ogura, H, et al. Impact of blood glucose abnormalities on outcomes and disease severity in patients with severe sepsis: an analysis from a multicenter, prospective survey of severe sepsis. PLoS One. 2020;15(3):e0229919. doi:10.1371/journal.pone.0229919
51. Bersoux, S, Cook, CB, Kongable, GL, Shu, J, Zito, DR. Benchmarking glycemic control in U.S. hospitals. Endocr Pract. 2014;20(9):876-883. doi:10.4158/EP13516
52. Swanson, CM, Potter, DJ, Kongable, GL, Cook, CB. Update on inpatient glycemic control in hospitals in the United States. Endocr Pract. 2011;17(6):853-861. doi:10.4158/EP11042
53. Wasko, MC, Hubert, HB, Lingala, VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298(2):187-193. doi:10.1001/jama.298.2.187
54. Schultz, O, Oberhauser, F, Saech, J, et al. Effects of inhibition of interleukin-6 signalling on insulin sensitivity and lipoprotein (a) levels in human subjects with rheumatoid diseases. PLoS One. 2010;5(12):e14328. doi:10.1371/journal.pone.0014328
55. Marfella, R, Paolisso, P, Sardu, C, et al. Negative impact of hyperglycaemia on tocilizumab therapy in Covid-19 patients. Diabetes Metab. 2020;46(5):403-405. doi:10.1016/j.diabet.2020.05.005
56. Petrauskiene, V, Falk, M, Waernbaum, I, Norberg, M, Eriksson, JW. The risk of venous thromboembolism is markedly elevated in patients with diabetes. Diabetologia. 2005;48(5):1017-1021. doi:10.1007/s00125-005-1715-5
57. Stefan, N, Birkenfeld, AL, Schulze, MB. Global pandemics interconnected-obesity, impaired metabolic health and COVID-19. Nat Rev Endocrinol. 2021;17(3):135-149. doi:10.1038/s41574-020-00462-1
MAR-0000465 Rev 1.0