Publication
Journal of Diabetes Science and Technology
Date
January 2018
Authors
Bruce Bode,1 John G. Clarke,1 Joseph Johnson1
ABSTRACT
Purpose: The purpose was to improve the quality of care of at-risk patients through the addition of connected BG meters and CDSS to improve workflow and thus provide more efficient titration of patient’s insulin regimens remotely between office visits in an attempt to treat them to their glucose targets faster and efficiently, and maintain that improvement over time.
Methods: Hardware and software included a real-time cellular-enabled blood glucose (BG) meter and Glytec’s Glucommander™ clinical decision support software (CDSS). A quality improvement (QI) project with retrospective before-and-after comparison was conducted. The training period was 90 days and then the project ran for another 11 months. A protocol comprised Glytec CDSS software, which recommends titration intervals from 3 to 28 days as a function of glycemic control, specifically, longer intervals for better control. There were 46 clinic patients.
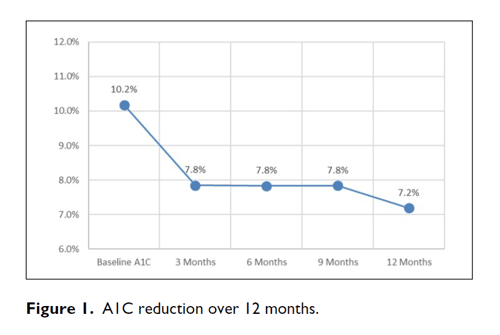
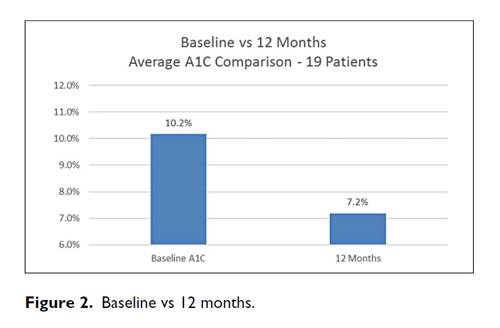
Results: A1C decreased from a baseline average of 10.2% to 7.8% at 3 months, 7.8% at 6 months, 7.8% at 9 months, and 7.2% at 12 months. The baseline-to-final A1C decrease shows a P <.00001 by paired t-test. Out of 36 315 BGs, the average number of BG tests per day was 3.03 during the first 3 months and 2.47 during the final 3 months. The percentage of BGs <54 mg/dL was 0.33% and the percentage of BGs <40 mg/dL was 0.05%.
Conclusion: This QI project demonstrated the use of CDSS including its built-in feature of titration interval recommendation can safely and effectively lower A1C for at-risk patients, treat patients to target safely, and maintain those improvements over 12 months of follow-up.
BACKGROUND
Due to the progressive deterioration of beta-cell function over time, the natural history of diabetes often necessitates the addition of insulin therapy. Unfortunately, insulin initiation remains difficult and attrition rates remain high; with the estimated probability of insulin discontinuation being 82.0% in the first year, 61.5% in the first 90 days, and 41.0% in the first 31 days.1 In addition, evidence suggests that patients often remain on low and ineffective doses of insulin and that their insulin doses are not adjusted sufficiently to achieve treatment targets.2 There are several reasons for not intensifying the insulin regimen both from the provider not having time to titrate the basal and bolus insulin as well as the patient not being fully educated in insulin management and the need to do frequent blood glucose (BG) measurements. In addition, there are insufficient adult endocrinologists to satisfy the current and future demand for experts in diabetes management.3 Even with the introduction of dozens of medications to the market,4 the percentage of patients with an A1C above 9% and the percentage of patients not at A1C goal has changed little in the last decade.5-7 Thus, technology is needed to help patients better self-manage their diabetes using basal and bolus insulin therapy. Numerous clinical decision support products (apps and software) promoting insulin dose titration have been FDA cleared in the last year.
Glucommander™ by Glytec Greenville, SC is an FDA-cleared and CE marked class II medical device utilized as the clinical decision support software (CDSS) for insulin dose titration in our quality improvement (QI) project. Its efficacy has been well documented8,9 and has been used to treat tens of thousands of hospitalized patients over the last five years. The ability for CDSS to move to the outpatient environment was enabled through technologies such as cellular and Bluetooth-capable BG meters. CDSS provides integrated and personalized diabetes therapy management intended to assist health care providers with subcutaneous insulin dose titration by analyzing BG data and calculating patient-specific insulin recommendations for basal, mealtime, and correction doses. Once patients achieve their target glucose range, CDSS recommends an increased time interval between interventions with continued remote monitoring with the use of integrated cellular and Bluetooth-enabled BG meter partners. With this expansion to the outpatient environment, CDSS is now available to manage patient’s insulin titration across the entire continuum of care.
PURPOSE
The purpose was to improve the quality of care of at-risk patients through the addition of connected BG meters and CDSS to improve workflow and thus provide more efficient titration of patient’s insulin regimens remotely between office visits in an attempt to get them to their individualized glucose targets faster and efficiently, and subsequently maintain that improvement over time.
STUDY DESIGN
This study employed a retrospective paired before-and-after design without a control group. The intervention is a system involving the addition of a cellular-enabled BG meter and insulin dose titration guided by Glytec CDSS, with scheduled titration intervals of 3, 7, 14, or 28 days.
METHODS AND MATERIALS
Software
Glytec CDSS is an FDA-cleared cloud-based clinical decision support tool utilized by a health care provider to assist with insulin dose titration through the recommendation of basal and/or bolus dose changes based on aggregated BG data over a 3-, 7-, 14-, or 28-day period.
Hardware
The hardware was a Telcare cellular-enabled BG meter. Telcare is a BioTelemetry Company, Concord, MA.
Protocol
A QI project adding cellular-enable BG meters and CDSS in an attempt to help at-risk patients achieve better glucose control. The software was operated by a nurse CDE (nCDE) at time-intervals of 3, 7, 14, or 28 days to assist with remote insulin dose titration between office visits. The interval choice was recommended by the software as a function of glycemic control wherein new patients and patients with higher BGs are given shorter intervals, which are relaxed as better BG control is achieved. Communications of changed doses to the patient were by email, telephone, text messages to the patient’s cell phone, or text message to the cellular-enabled BG meter.
PATIENTS
The project comprised 46 patients (see Table 1) with type 1 or type 2 diabetes requiring insulin, ages 18 and above, nonpregnant, able to self-manage their diabetes (finger sticks, insulin injections, and treatment of hypoglycemia), and willing to test BG 4 times a day.
All patients participating in the QI project were from a single diabetes center. These patients were referred to the nCDE from health care practitioners in the center to improve their glycemic control using both basal and bolus insulin therapy. No formal consent was obtained, and no IRB was involved or required.
The metrics and demographics of these 46 patients are listed in Table 1.
Table 1. Metrics, Demographics
| Metric | Parameter | ± SD |
|---|---|---|
| Average Age, years | 57.3 | 14 |
| Male, n (%) | 29 (63%) | |
| Average Initial BMI, kg/m2 | 31.2 | |
| Average Final BMI, kg/m2 | 33.0 | |
| Change to Average BMI, kg/m2 | 1.7 | |
| Average Initial Weight, kg | 93.8 | 52.7 |
| Average Final Weight, kg | 96.3 | 53.0 |
| DM type 2, n (%) | 35 (76%) | |
| DM type 1, n (%) | 11 (24%) | |
| Average Years with DM | 16 | 13 |
TREATMENT AND ASSESSMENTS
The CDSS was initially ordered by the provider at the time of an in office visit, via an order set which included the insulin(s) to be used, the starting insulin dose(s) (either weight-based or custom doses), the target glucose range (100-140 mg/dL) and the initial dose titration interval (3 days).
All BG data were collected using the Telcare cellular-enabled BG meter, which was able to provide near real-time glucose results into a secure cloud environment that then interfaced into the CDSS.
The nCDE enrolled the patient on the CDSS based on the provider’s orders, activated, registered and educated the patient regarding the use of the cellular-enabled BG meter, and advised the patient to self-monitor BG before meals and at bedtime. An individualized meal plan, emphasizing similar size carbohydrate portions was discussed. Subsequently the nCDE would utilize the CDSS daily during workdays to scan for low BG readings and to adjust insulin doses when the patient was due a titration. After the initial three-day titration, the CDSS would recommend new insulin doses as well as a new dose titration interval of 3, 7, 14, or 28 days based on the patient’s glucose control.
Updated insulin doses were sent to the patient, either through the email feature in the CDSS, by using the meter’s messaging system onto the display of the patient’s cellular-enabled BG meter, via a mobile text message, or calling the patient on the telephone. The mode of communication was based on the patient’s preference.
Primary and Secondary Endpoints Evaluated
The primary efficacy endpoint was the change in A1C from baseline to months 3, 6, 9, and 12 after the initiation of the new workflow and technologies. Secondary and other efficacy endpoints included time to glucose target, time to glucose <180 mg/dL. The number of hypoglycemic events was used to evaluate safety. Hypoglycemia was defined as BG <54 mg/dL and severe hypoglycemia was defined as BG <40 mg/dL.
Statistical Analysis
The project was a paired before-and-after design. Average number of BG tests/day was calculated at 3 and 12 months. Average A1C was calculated at 3, 6, 9, and 12 months. Average overall percentage was calculated for hypoglycemia (<54 mg/dL) and severe hypoglycemia (<40 mg/dL). The baseline-to final A1C drop (3 to 12 months) was analyzed by paired t-test.
RESULTS
During treatment with CDSS, A1C decreased from a baseline average of 10.2% to 7.8% at 3 months, 7.8% at 6 months, 7.8% at 9 months, and 7.2% at 12 months (Figures 1 and 2). The baseline-to-final A1C decrease shows a P <.00001 by paired t-test. Hypoglycemia was infrequent throughout (see Table 2). Out of 36,315 BGs, the average number of BG tests per day was 3.03 during the first 3 months and 2.47 during the final 3 months. The median time for patients to achieve three consecutive days with their average daily BGs <180 mg/dL was 7 days. As a backup check the mean BG was tested also; it declined from 214 mg/dL to 162 mg/dL with P <.00001. This drop is free from regression toward the mean.
Table 2. Results
| Metric | Parameter | ±SD |
|---|---|---|
| Number of Patients | 46 | |
| Median #Days Until Day-Averaged BG <180 mg/dL for 3 Consecutive Days | 7 | |
| % BGs <54 mg/dL | 0.33% (126) | |
| % BGs <40 mg/dL | 0.05% (18) | |
| Number of BGs | 36,315 | |
| Insulin doses | ||
| Average Initial TDD, units/kg | 0.66 | 0.34 |
| Average Final TDD, units/kg | 0.91 | 0.52 |
| Average Initial % Basal Insulin | 50% | |
| Average Final% Basal Insulin | 46% | |
| Change to Average Weight, kg | 2.5 |
DISCUSSION
This QI project in at-risk patients failing insulin therapy showed that with the addition of new technologies (cellular-enabled BG meters and CDSS) it is possible to safely and effectively get patient to their glucose targets while also improving the efficiency and workflow of the care team to allow for remote insulin titration between office visits. After the provider’s order of an initial dose and glucose target the nCDE was easily able to incorporate the CDSS into their daily workflow to be able to titrate basal and/or bolus insulin doses to dramatically improve glycemic control with minimal hypoglycemia for a group of patients.
Patient referrals to the project were made by multiple prescribers in the office and often took place during or immediately after the scheduled office visit. All education, supplies, and medicines were provided on the spot, and the patient left the office under the new treatment regimen. The initial visit lasted on average one and a half hours. The CDSS workstation was designed for efficiency. The time spent daily to sign on and check for low BG’s was minimal on 46 patients, since low BGs were flagged by the software. Those scheduled for insulin adjustments took an average of five minutes per patient to review, communicate new doses, and chart. Since the encounters were driven by the patient’s progress, the number of titrations varied from day to day.
There are several limitations of this observational study:
- Patients were all from one diabetes center.
- There was no control group.
DISCUSSION OF LIMITATIONS IN RELATION TO THE PURPOSE
The purpose was to improve the quality of care of at-risk patient through the addition of connect BG meters and CDSS to improve workflow and thus provide more efficient titration of patient’s insulin regimens remotely between office visits in an attempt to get them to their individualized glucose targets faster and efficiently, and subsequently maintain that improvement over time. In this role, the intervention of the QI project is defined as the use of the addition of cellular-enabled BG meters and CDSS to provide for efficient insulin dose titration between office visits, this included variable frequency of titration as described. When the results of this QI project are judged against this purpose, the goals have clearly been met.
Many of the elements of diabetes control, including meters, lab tests, numerous medications, diabetes self-management education (DSME), and knowledgeable practitioners have been available for years; however, glycemic control for patients has not appreciably changed.5-7 Effectively adding new technologies to improve the current delivery of care was well demonstrated by this project’s ability to bring together many parts of the diabetes ecosystem (provider, patient, care team, DSME, connected BG meter, and CDSS) to successfully treat patients to their glucose and A1C goals and maintain that control. This project would suggest that scaling a specialty practice in this manner could assist with the management of the many patients in such dire need of better control that we would argue requires this type of multi-pronged approach.
CDSS used by CDEs, pharmacist, or even other clinicians such as LPNs and RNs under their guidance with the prescribers atop the care pyramid, can enhance workflow and scalability of resource-restricted entities. It can be of benefit in many different work environments. It can assist with the shortage of specialists and in the setting of a chronic disease management center, it can allow clinical expertise to be scaled to serve a much larger number of patients. While in the primary care setting, CDSS can afford a level of expertise that the provider may otherwise not possess. Therefore, it seems that CDSS can help to alleviate a number of the concerns outlined in the introduction:
- Insulin attrition1
- Ineffective doses of insulin and insufficient dose adjusts2
- Insufficient adult endocrinologists to manage all the people with poorly controlled diabetes3
- percentage of patients with an A1C above 9%, which has changed little in the past decade5-7
The next iterations of CDSS will be related to continuous glucose monitoring as a data source and integrating smart insulin pen technology with their insulin data into the software. By further improving the care of patients across the continuum and better managing the transitions of care with CDSS, we would expect to see positive effects on glycemic control, patient satisfaction, readmissions, ED visits, patient outcomes, and cost.
CONCLUSION
This QI project demonstrated the use of CDSS including its built-in feature of titration interval recommendation can safely and effectively lower A1C for at-risk patients and maintain that improved control for a year. The project demonstrates the safety, efficacy, and workflow improvements for patients and the health care team through the use of connected BG meters and CDSS to titrate insulin doses between office visits, get patients to target safely, and maintain those improvements over 12 months of follow-up.
Abbreviations
BG, blood glucose; CDSS, clinical decision support software; DSME, diabetes self-management education; MDI, multiple daily injections; nCDE, nurse CDE; QI, quality improvement.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JGC is an employee of Glytec. BB holds stock in Glytec and is a Glytec scientific advisory board member.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
AFFILIATIONS
- Atlanta Diabetes Associates, Atlanta, GA, USA
REFERENCES
- Ascher-Svanum H, Lage M, Perez-Nieves M, et al. Early discontinuation and restart of insulin in the treatment of type 2 diabetes mellitus. Diabetes Ther. 2014;5(1):225-242.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin com-pared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837-853.
- Vigersky RA, Fish L, Hogan P, et al. The clinical endocrinology workforce: current status and future projections of supply and demand. J Clin Endocrinol Metab. 2014;99(9):3112-3121.
- Food and Drug Administration. Type 2 diabetes U.S. drug approvals: 2005-2015. Available at: https://www.accessdata.fda.gov/scripts/cder/drugsatfda/. Accessed August 28, 2017.
- Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med. 2013;368(17):1613-1624.
- National Committee for Quality Assurance. The state of health care quality 2015. Available at: http://www.ncqa.org/ReportCards/HealthPlans/StateofHealthCareQuality.aspx. Accessed April 20, 2016.
- Carls GS, Huynh J, Tuttle E, Edelman SV. Achievement of glycated hemoglobin goals in the U.S. remains unchanged through 2014. Poster presented at: American Diabetes Association 76th Scientific Sessions; June 10-14, 2016; New Orleans, LA.
- Newsom R, Patty C, Camarena E, et al. Safely converting from sliding scale to basal bolus insulin across an entire medical center via implementation of the eglycemic management system. Poster presented at: American Diabetes Association Scientific Sessions; June 2017; Chicago, IL.
- Aloi J, Ullal J, Chidester P, McFarland R. “AUTO Study” automatic titration to target: subcutaneous basal bolus insulin management using eGMS in the non-ICU setting. Poster presented at: American Association of Clinical Endocrinologists Annual Scientific and Clinical Congress; May 4, 2017; Austin, TX.