Subcutaneous Insulin Dosing Calculators for Inpatient Glucose Control
Publication
Current Diabetes Reports
Date
November 2019
Authors
Jagdeesh Ullal,1 Joseph Aloi2
ABSTRACT
Purpose of Review: The goal of this review is to summarize information about insulin dosing software and calculators used as computerized decision support systems or electronic glucose management systems (eGMS). These are used for hospitalized, insulin-treated patients with diabetes. We describe the advantages and disadvantages and the rationale for their use.
Recent Findings: We compared commercially available insulin dosing software, namely, Glucommander™, EndoTool®, GlucoStabilizer®, and GlucoTab®, in addition to computerized order entry systems that are available in electronic health records. The common feature among these eGMS is their ability to limit occurrences of hypoglycemia while achieving and maintaining patients at target blood glucose level.
Summary: More research needs to be done examining the efficacy of eGMS in disease-specific states and their benefits and utility in preventing adverse outcomes. Their long-term benefits to health care systems are beginning to emerge in cost-saving benefits and prevention of readmissions.
INTRODUCTION
Electronic or computer decision support systems (CDSS) that support the management of glucose control in the inpatient setting have been in use for well over a decade and have become a mainstay in many health care institutions [1]. Electronic glucose management systems (eGMS) are varied in their complexity as well as design and features. Following the American Diabetes Association and the Endocrine Society’s development of guidelines for the management of inpatient hyperglycemia with basal-bolus insulin, multiple institutions and health care systems have initiated the utilization of algorithmic glucose management to assist in managing inpatient hyperglycemia [2•, 3-6]. Studies have demonstrated that
basal-bolus therapy achieves target blood sugars in a more consistent fashion compared with sliding scale insulin treatment. This was clearly demonstrated in the Randomized Study of Basal-Bolus Insulin Therapy (RABBIT) trials for both medical and surgical patients [6, 7]. While sliding scale-based insulin therapy has gradually given way to basal-bolus management as a best practice in the management of inpatient blood glucose, treatment gaps still exist with subcutaneous basal-bolus
management [8]. Debate continues as to the appropriate goals of glucose control in the inpatient setting following the publication of the landmark article delineating the benefits of glucose management in critically ill patients [9]. The Normoglycemia in Intensive Care Evaluation–Survival Using Glucose Algorithm Regulation (NICE SUGAR) study called into question the reported benefits of tight blood sugar control in critically ill patients and recommended changing targets for blood sugar control in the critical care setting due the high incidence of hypoglycemia seen when attempting “tight” blood glucose control [10]. This resulted in consensus that a safe glycemic target for critically ill patients is between 140 and 180 mg/dL while non-critically ill patients benefit from targets of 100 to 140 mg/dL or random blood sugars of under 180 mg/dL, but questions remain as to which insulin strategy is best for non-critically ill patients [11].
Basal-bolus insulin treatment closely mimics the physiologic kinetics of insulin during the fasting state as well as postprandial state. Basal-bolus insulin is better capable of managing glucose variations with meals and in the fasting state. The pancreatic beta cells are continuously releasing insulin to maintain euglycemia, and there is postprandial surge in insulin to deal with glucose excursions after meals. This forms a fundamental basis of utilization of basal insulin in combination with rapid-acting insulin in the hospital setting. Sliding scale insulin is a reactive approach to treatment of hyperglycemia but is in wide use due to its simplicity and convenience for nursing staff and/or house staff despite data discouraging its use [12]. Meta-analysis comparing sliding scale insulin and basal-bolus therapy have not clearly endorsed the benefits of one strategy versus the other. While there appears to be a slightly higher risk for hypoglycemia and total daily insulin dose in basal-bolus therapy, sliding scale insulin prolongs length of hospital stay and is associated with higher blood sugar trends [11]. Thus, there is a clear institutional benefit in mandating the use of basal-bolus insulin for the management of hyperglycemia in hospitals.
Following the enactment of the Federal Health Information Technology for Economic and Clinical Health (HITECH) act in 2009, hospitals began to engage with the terms of “meaningful use” and utilize protocol-driven insulin order sets that are structured around basal-bolus insulin [13]. Subcutaneous insulin protocols for the management of basal-bolus insulin have thus burgeoned across specialties, hospitals, and health care institutions varying from clinical unit-specific protocols to the use of generalized protocols across an entire institution [14, 15]. There have been concerted efforts across a variety of teaching and non-teaching hospitals to begin implementation of basal-bolus insulin protocols [16, 17]. The Centers for Medicare and Medicaid Services began the recognition of certification for inpatient diabetes management (Joint Commission, Advanced Certification in Inpatient Diabetes) [18]. Many institutions began to collect and analyze glucose-related information referred to as “Glucometrics” for the purpose of quality improvement and to potentially report to federal agencies [17, 19].
The American Diabetes Association now recommends the use of validated written protocols or computerized protocols for blood sugar management in its standards of diabetes care annual updates [20]. eGMS are now available that provide clinical decision support systems to dose both intravenous and subcutaneous insulin. In 2013, the Planning Research in Inpatient Diabetes (PRIDE) group suggested further action in the development and utilization of clinical decision support tools in achieving excellence and overcoming obstacle in inpatient glycemic control [21]. There have been previous comparisons of the various eGMS but these have predominantly focused on intravenous insulin dosing [22]. The purpose of this review is to focus on inpatient utilization of subcutaneous insulin dosing eGMS.
COMPUTERIZED ORDER ENTRY SYSTEMS
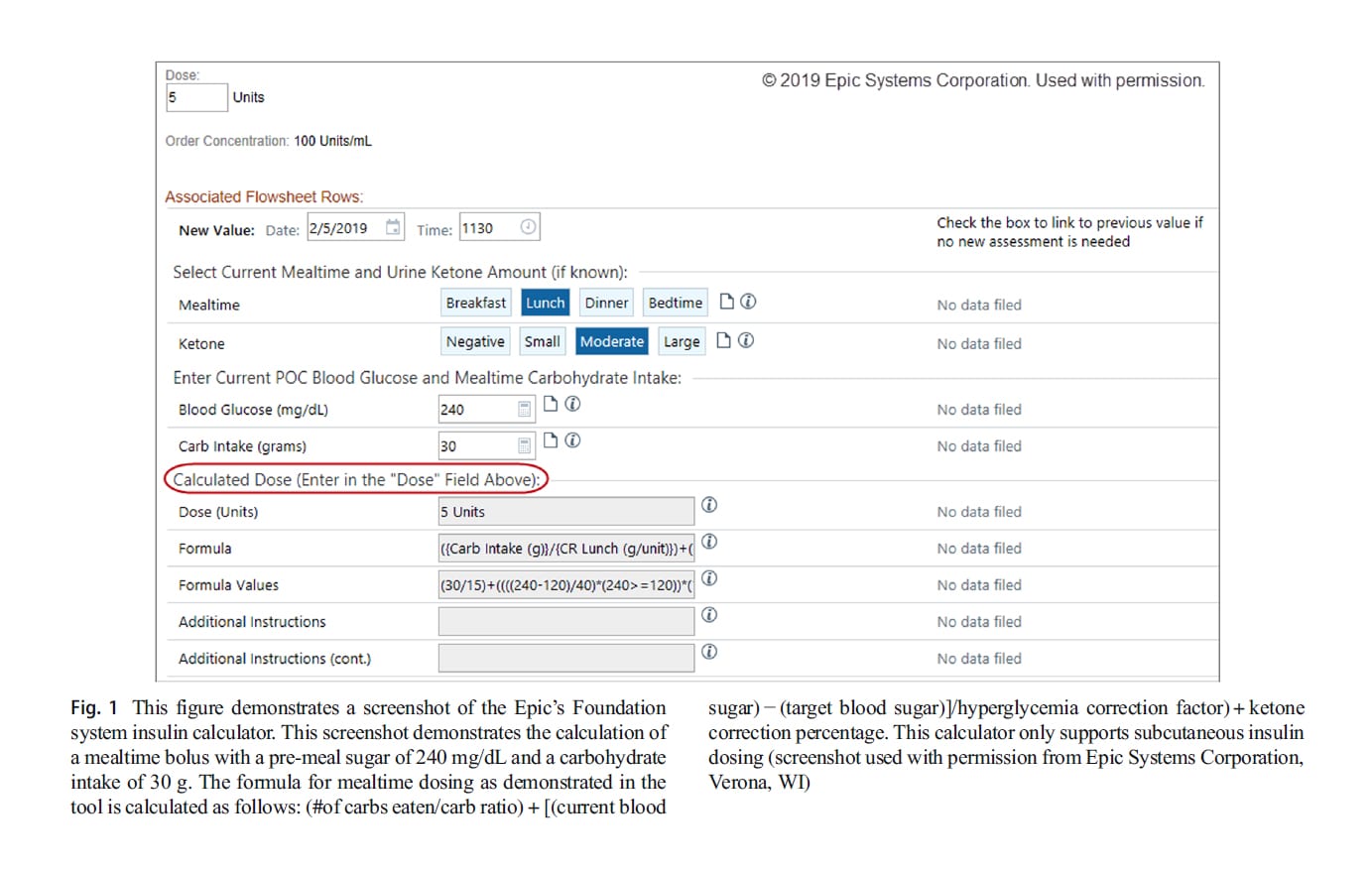
Some electronic health records (EHR) can be designed to provide order entry systems to dose basal-bolus insulin treatment. An example of an integrated basal-bolus order system is available in Epic (Epic, Verona, WI). Clinicians often calculate insulin doses for patients based on pre-meal blood glucose or based on carbohydrate intake. Electronic health records have partnered with various institutions to develop methods of calculating insulin doses. For example, the University of Michigan Foundation system insulin calculator uses dynamic calculations and precise dosing in addition to correction insulin based on the provided blood sugar goals. The calculator uses order-specific questions, rules, flowsheet groups, and rows and requires no external device or software-based calculator. In the Foundation system, the calculator uses the following values: blood glucose targets, current point-of-care blood glucose reading, hyperglycemia correction factors (insulin sensitivity factors), carbohydrate coverage ratios for breakfast, lunch, and dinner, bedtime, a.m. snacks, and p.m. snacks, and urine ketone correction percentage (moderate ketones and large ketones). Separate targets can be set for daytime and night glucose levels and correction ratios. The formula that is used in the Foundation system uses the number of carbs eaten, the bicarbonate level, the current point-of-care blood sugar, the pre-set target blood sugar, and it factors in ketone if present. This data is drawn from the laboratory interface of the EHR and there is no need for manual entry. The implementation of the above system in pediatric patients has stream- lined ordering and administration of insulin while promoting safety and user satisfaction [23]. A screenshot of a version of the Epic diabetes dosing tool is illustrated in Fig. 1.
The SQ insulin CDS tool developed at Johns Hopkins is another example of an Epic-based “smart form” that is a pre-populated form which abstracts patient-specific electronic data from the health record. It obtains body weight, type of diabetes, home insulin doses, and nutritional status and uses this information to calculate total daily insulin doses, distributing them into basal and bolus insulin doses. A custom-made correctional scale is also generated. This tool is integrated into the admission workflow and is embedded in the EHR. It does not require installation of any other software [24]. The Cleveland clinic implemented a decision support system for the use of concentrated or U-500 insulin and was able to reduce insulin dosing errors by 50% [25].
COMPUTER-BASED ELECTRONIC DECISION SUPPORT SYSTEMS FOR GLUCOSE MANAGEMENT
Basal-bolus insulin therapy is rooted in an algorithmic process based on patient weight, insulin sensitivity, carbohydrates consumed, and other clinical factors. Most of these clinical parameters can be easily transferred into an electronic format followed by EHR-embedded software to assist in prompting the provider with insulin dose recommendations and dose changes.
The implementation of basal-bolus insulin therapy is subject to potential points of failures from time of admission of patient to the hospital to the time the patient receives insulin. The factors that influence this include human error, lack of knowledge of insulin or the effects of basal-bolus treatment, lack of experience with insulin, extremes of insulin resistance or sensitivity, management of patients by multiple clinical teams, mismatch of food and insulin doses, clinical inertia, and rapidly changing clinical conditions in the hospital. There is a general perception that basal-bolus therapy is complex and therefore not easily carried out by nursing staff or admitting teams. The limitations of clinician-based glucose management are failure to adhere to blood glucose targets, overemphasis on the fear of hypoglycemia due to lack of knowledge, lack of individualization of treatment and skepticism of keeping patients’ in the target range, and clinical inertia in changing insulin doses from day to day.
Several eGMS are now available to guide basal-bolus insulin therapy which are FDA-cleared. In general, they are indicated for patients with type 1 and type 2 diabetes and patients with hyperglycemia who are treated with insulin [26]. Table 1 lists the commercially available software marketed for the management of subcutaneous insulin dosing and contains a brief comparison. These “devices” or software algorithms are particularly helpful to hospital systems that do not have expertise to manage inpatient diabetes (endocrinologist or glucose management teams). eGMS provide real-time assistance to dose insulin while displaying glucose trends and glucose values. Some eGMS have the ability to gather Glucometric data and provide them as a report on a periodic basis [27]. There is an initial and recurring financial investment that hospital systems have to commit to purchase these software programs and the need for upfront staff education to establish new workflows. Most eGMS make dose recommendations for hyperglycemia and some have the ability to make treatment recommendations for hypoglycemia correction.
Most subcutaneous insulin dosing calculators are based on pre-meal blood sugar capillary measurements usually 3 times daily and once at bedtime. Some systems allow an as-needed blood sugar measurement based on provider discretion. Most calculators have auto-population of details from the health record including demographics and anthropometric details such as weight and height. Some utilize laboratory measurements such as hemoglobin A1c and creatinine level to calculate dosing whereas others utilize these numbers for purely informational purposes. The total daily dose is based on the patient’s weight or previous experience from the outpatient setting. The total daily insulin dosing is then divided into basal insulin and bolus insulin doses. The bolus insulin is further divided into 3 doses for break- fast, lunch, and dinner. The basal insulin is dosed either in the morning or at bedtime or both. Mealtime dosing can be changed based on the percent of meal eaten or carbohydrates consumed. Correctional insulin or supplemental insulin is dosed based on the chosen target, e.g., 100-140 mg/dL or 120-160 mg/dL. The correctional dose is usually based on patient weight or provider judgment based on the insulin sensitivity. Above specifications can be modified by providers based on the clinical situation. If a meal is missed but a pre-meal blood sugar is obtained, then a correction-only dose is administered.
SAFETY OF EGMS
For the successful implementation of eGMS in any health care system, it is recommended that this be done in a carefully planned approach with approval and acceptance from all parties involved. The safety of eGMS depends on adequate education and training of clinicians (emergency providers, admitting providers, and specialists), nursing, house staff, pharmacy, and ancillary health care personnel. Evidence shows that if safely incorporated into inpatient clinical practice, the use of eGMS can yield improved patient care, achieving safe glucose targets and financial returns by reducing length of stay and reducing burden of nursing care in addressing severe hyperglycemia or hypoglycemia treatment and possibly long-term benefits such as reduced readmission [2•, 28•, 29].
Glucommander™ subcutaneous (SubQ) has a well-validated algorithm that leads to decreased rates of hypoglycemia and improvements in rates of hyperglycemia by providing real-time dose changes from meal to meal (bolus dosing) and day to day (basal dosing). There are several safety features built into this software including nurse verification of doses, reentry of glucose that were auto-populated from the EHR, missed dose reminders with alarms, entry of mealtime carbohydrate or percent eaten to adjust the dosage of mealtime insulin, and hypoglycemia correction algorithms. If correction insulin doses are ordered, then the software requires a current blood glucose (BG). We have previously published retrospective data from 9 hospital systems for non-critically ill patients on Glucommander™ SubQ with a target of 140-180 mg/dL. This was obtained on 1687 patients over a 6-month time frame. There was a 0.08% incidence of hypoglycemia under 40 mg/dL. There was an 11.2% occurrence of hyperglycemia above 250 mg/dL. The average duration of time during which the program was utilized was 5.9 days [30]. In a study with comparison of 13,351 patients treated with eGMS and 45,335 patients treated with usual standard of care utilizing target blood sugars of 100-180 mg/dL, the incidence of patients experiencing any hypoglycemia <70 mg/dL was 13.8% for eGMS compared with usual care (UC) at 21.7% (P ≤0.01). The eGMS had 6% <60 mg/dL and 2.5% <50 mg/dL compared with UC at 13.7% (P ≤0.01) and 7.7% (P ≤0.01). The eGMS also had less severe hypoglycemia at 0.9% of patients with a BG <40 mg/dL compared with UC at 3.6% (P ≤0.01). The average BG for patients treated on eGMS was 178 mg/dL compared with UC at 188 mg/dL. Overall, eGMS had a lower number of patients with hypoglycemia and lower average BG during hospitalization compared with standard of care. This indicates that the usage of eGMS is relatively safe and yields better outcomes in terms of the goals for glucose control [31].
The GlucoStabilizer® also demonstrates a reduction in average time to reach glycemic target, reduced frequency of hypoglycemia, and an increased number of patients achieving target glycemic goals [22]. Previously published data indicates that the average blood sugar was around 158 mg/dL with 40% of blood sugars in the set target of 100–150 mg/dL and about 30% of blood sugars above 180 mg/dL. The severe hypoglycemia rate (<40 mg/dL) was 0.18% [32]. The program issues warnings for potentially unsafe insulin doses. Basal insulin doses ≥100 units and bolus insulin doses ≥30 units required physician confirmation. Alerts for hyperglycemia are created when a single BG exceeds 350 mg/dL or two consecutive BG exceed 220 mg/dL. Warning thresholds can be adjusted to a provider or institution preferences.
The EndoTool® software is an FDA-cleared class II medical device that provides precise clinical decision support for any situation where subcutaneous (SubQ) insulin is administered, including pediatrics. It has data supporting used in intravenous insulin but lacks published data comparing the efficacy of subcutaneous dosing software to paper protocols or standard care [33].
The GlucoTab® software when compared with a paper-based workflow protocol demonstrated decreased errors with insulin dosing. There was an increased risk of hypoglycemia in the group treated with paper-based protocol when compared with GlucoTab® software. Workflow deviations in the paper-based group had increased hyperglycemia implying that automated handling of blood sugars by the software had a potential to reduce risks of insulin, avoiding hypoglycemia or hyperglycemia [34]. In an open, non-controlled intervention study with GlucoTab®, it was found that 50.2 ± 22.2% were in the target range of 70-140 mg/dL. Most physicians adhered to the eGMS suggesting total daily insulin doses in 97.5% of cases. There were no severe hypoglycemic events below 40 mg/dL. The study allowed manual correction of BG levels and insulin doses and belated entry of values with a time stamp. The GlucoTab® considered the amount of bolus insulin that was active from the previous dose and reduced boluses by 25% akin to insulin-on-board features present in insulin pump therapy. There was improved nurse adherence and less frequency of missed insulin doses [35].
Table 1. Comparison of Commercially Marketed eGMS Tools
| eGMS Software | Company | Electronic Platform | Integration into EHR | Enteral and Parenteral Calculation | Other Analytic and Software Additions | Discharge Recommendations |
|---|---|---|---|---|---|---|
| Glucommander™ | Glytec Greenville, SC |
Cloud-based integration. | Yes | Yes | Glycemic management information (SmartClick®), early patient identification (GlucoSurveillance®), and data analysis (GlucoMetrics®). |
Hospital-to-home tool used to recommend post-discharge therapy. |
| EndoTool System® | MD Scientific LLC Charlotte, NC |
Citrix server-based integration. | Yes | Yes | EndoTool Analytics. | |
| GlucoStabilizer® | Medical Decision Network Charlottesville, VA |
Secure server system. | Yes | No | Inbound ADT (Admit, Discharge, Transfer) and outbound HL7 information. |
|
| GlucoTab® | Joanneum Research GmbH Graz, Austria |
Mobile, tablet-based client- server system. This system is Wi-Fi based. |
Yes | No | HL7 ADT interface for patient data keeps ward patient information up to date. HL7 laboratory data interface. |
Home therapy and prescribed therapy can be transferred via the interface. |
BENEFITS OF EGMS
One of the standards for measuring efficacy of eGMS is time-to-target and time-in-range for BG. Time-to-target represents the time it takes for the software-based algorithmic dosing to get BG in the target range. Time-in-range represents the time that the patient’s BG were maintained in the predetermined glucose range. In a retrospective study examining 5718 patient on basal-bolus insulin–treated patients, the prescribed target was achieved 0.8 days on average and the glucose levels were maintained in target during the hospital stay with very limited mild or severe hypoglycemia [36]. While comparing paper protocols and Glucommander™, there were a total of 32,306 (67.59%) blood sugars in the 70-180 mg/dL in the Glucommander™ group compared with 58,013 (60.97%), which was significant with a length of stay of 5.51 ± 3.19 days for the Glucommander™ group and 8.69 ± 4.89 days for the conventionally treated patients [28•].
A significant reduction in hypoglycemia is a decisive benefit of the use of eGMS when compared with paper-based protocols. This is possibly due to elimination of manual insulin dosing calculation errors [35]. eGMS thus provide the benefit or more intensive glucose control without significant hypoglycemia. Every hypoglycemic episode bears a burden on the patient and the care team. Apart from the morbidity and mortality associated with hypoglycemia, there is a prolongation of hospital stay [37]. Hypoglycemia episode needs treatment, documentation, and follow-up care from nursing. There is provider and pharmacy involvement in the need to change dosing of insulin in response to hypoglycemia. Thus, the benefits of eGMS may be beyond just hypoglycemia reduction and may influence care team work satisfaction as it pertains to care of insulin-dosed patients with diabetes.
With subcutaneous insulin regimens that yield better glucose control with the use of eGMS, a safe insulin discharge regimen can be created for discharge. We have previously published a reduction in length of stay for patients admitted with stroke needing subcutaneous insulin using Glucommander™ compared with paper protocol [38]. The use of eGMS had a significantly lower readmission rate compared with standard care in patients undergoing coronary artery bypass graft surgery and other cardiac procedures [29].
BARRIERS AND RECOMMENDATIONS ON THE USE OF EGMS
The adoption of computerized decision support systems has received variable acceptance and utilization [39]. There is evidence to suggest that in general implementation of a CDSS works better in fine-tuning of therapy rather than trying to attempt to provide treatment choices and works better in institutional implementation rather than in ambulatory settings [40]. However, these notions have not been necessarily borne out in the use of eGMS. There are numerous barriers to the implementation of electronic glucose management systems. These can broadly be classified into 4 categories: organizational barriers, end user barriers, patient issues, and lastly barriers pertaining to software and industry.
The problems within an organization or institution include both hardware and software availability or lack thereof. Organizations may often have to invest in additional hardware and other equipment to facilitate the utilization of eGMS. There may be the need for dedicated terminals or a sentinel terminal such as a personal computer or laptops or mobile devices or the need for additional servers to store and process data to connect to cloud interfaces. The ability of eGMS to integrate with electronic health record limits the end user experience. There is thus a need to have versatile EHR that integrate with eGMS and vice versa. Most commercially available eGMS software claim easy integration into existing EHR. From an organizational standpoint, the initial implementation process can often be a crucial step in the adoption of a new technology. Our recommendation is that the process be implemented in a phased approach, one unit at a time or one hospital at a time in larger health care organizations. There needs to be a core group of staff who are trained and who can train other trainers in the use of the software program or devices. “Champions” are designated individuals who can shepherd the process of implementation, and we recommend the use of both nursing champions and provider champions. Often champions may be needed in various departments such as emergency medicine, hospitalists, and critical care. The utility of inpatient diabetes educators can be vital in the process. A nursing unit champion can also be designated for day shift and night shift. There may be a need to hire separate training staff or contract with temporary trainers. Clear delineation of roles in the set-up, implementation, and execution processes of eGMS is important. From a financial standpoint, there is often an initial cost of eGMS purchase and recurring financial costs in the form of annual license or warranty renewals. The maintenance of privacy will be of concern for organizations that might have to share protocols and other know-how with eGMS. Varying adoption and use of software within the same health care organizations such as one unit to another, one department to another, or one hospital to another can be challenging. Large-scale implementation will need the wholehearted support from administration. A lack of systemic oversight of the roll-out process by glycemic steering committees or patient safety committees can lead to poor coordination. Close integration with the information technology departments is needed. Integration of the organizations’ laboratory and pharmacy software systems is going to be important in the flow of glucose and medication data into eGMS.
End users are defined as nurses, providers (physicians or mid-level staff), or trainees such as resident, fellows, or students. A lack of education prior to and after implementation can be a major barrier to the use of eGMS. There is often a perceived threat to professional autonomy and a perception of relinquishing control. Without adequate training, there will be a lack of understanding regarding the benefits and utility of eGMS. The implementation may be conceived as administrative heavy handedness and perceived as harm to patients. Lack of cooperation of staff from various levels such as pharmacy, nurses, and providers can largely be avoided with proper education. Lack of customization for different patient types may be a source of poor uptake among providers.
Patients on whom these software programs are used on do not often realize or may not be adequately informed about their use and utility. Opt-out options may be difficult to implement and will have to carefully be incorporated into treatment consents. The trust factor in providers or hospitals using or not using eGMS may play a role in where patients seek care. The perceptions of harm or lack of efficacy along with privacy concerns may have to be addressed on both provider and patient ends.
Lastly, inherent issues of eGMS include the inability to integrate into existing EHR, frequent prompts leading to provider fatigue, poor user interface, and difficulty with navigation. Integrated eGMS may have an advantage over stand-alone device or software programs. Many software programs are now available in the form of “apps.” A lack of availability in an “app” format or lack of ability to customize can hinder use. All eGMS need to have “down-time” workflow and options in the instance of power failure or lack of backup power generation. Lack of validation or evidence-based research to support the use of individual eGMS can be barriers to their use. Industry rules and regulations need to be followed. A failure of companies to comply with state and federal laws, rules, and regulations particularly in the area of patient privacy can jeopardize use. The vulnerability of malware attacks and the ease of malware embedment can jeopardize institutions’ EHR and information technology departments.
CONCLUSION
The complex problem of inpatient glycemic management has had a history of debates over best targets, approaches to management, and patient benefits from blood glucose control during acute hospitalization.
We have summarized the evidence supporting the adoption of basal-bolus insulin therapy for the general medical-surgical patient with anticipated benefits of decreased length of hospital stay and associated hospital complications.
The current manuscript provides the reader with a comprehensive review of the available EHR-embedded clinical sup- port devices for dosing insulin in the inpatient clinical setting. Our review suggests that use of eGMS is both safe and efficacious with rapid provider adoption. Use of eGMS can minimize the need for diabetes management experts at each hospital while standardizing care practices across a hospital system. The addition of eGMS protocols that provide both nursing and provider prompts when insulin orders are above pre-specified targets may help to minimize medical dosing errors as well as administration timing errors. Lastly, the ability of the EHR to port real-time data into the software analysis can help mitigate therapeutic inertia and prompt a dosing change linked to the changing clinical condition of the patient.
Use of eGMS has the potential to simplify the management of a traditionally difficult to manage patient population. Data support the improvements of hospital hypo- and hyperglycemia rates associated with the implementation of eGMS to manage basal-bolus insulin therapy. There are a wide range of products to select from including proprietary systems such as GlucoTab®, GlucoStabilizer®, EndoTool®, and Glucommander™ SubQ and EMR-embedded insulin dosing calculators available through Epic. The higher costs of the proprietary products may be offset by savings seen by de- creases in hospital costs and utilization, as we move towards a value-based reimbursement system. The use of insulin dose calculators already available within the EHR system Epic is 60 of 369 Epic organizations (a 16% adoption rate; Epic query 6/7/2019) suggesting a slow but steady increase in the use of provider-assisted clinical decision support for insulin prescribing.
In conclusion, the availability of eGMS should help providers achieve glycemic goals for patients with hyperglycemia with less effort and fewer clinical challenges. Ultimately it is hoped that these measures as they become more uniform across hospital systems will improve care, decrease overall costs, and minimize errors associated with insulin administration.
COMPLIANCE WITHE ETHICAL STANDARDS
CONFLICT OF INTEREST
The authors declare that they have no conflict(s) of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
AFFILIATIONS
- Center for Diabetes and Endocrinology, Division of Endocrinology, Department of Medicine, University of Pittsburgh Medical Center, Pittsburgh, PA, USA.
- Department of Internal Medicine, Section on Endocrinology and Metabolism, Wake Forest School of Medicine, Winston-Salem, NC, USA.
REFERENCES
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- Umpierrez GE, Hellman R, Korytkowski MT, Kosiborod M, Maynard GA, Montori VM, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol 2012;97(1):16-38.
- Aloi J, Bode BW, Ullal J, Chidester P, McFarland RS, Bedingfield AE, et al. Comparison of an electronic glycemic management system versus provider-managed subcutaneous basal bolus insulin therapy in the hospital setting. J Diabetes Sci Technol. 2017;11(1):12-6 This paper demonstrates the use and utility of eGMS compared with basal-bolus insulin therapy.
- Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119-31.
- Mulla CM, Lieb DC, McFarland R, Aloi JA. Tides of change: improving glucometrics in a large multihospital health care J Diabetes Sci Technol. 2015;9(3):602-8.
- Aloi JA, Mulla C, Ullal J, Lieb DC. Improvement in inpatient glycemic care: pathways to Curr Diab Rep. 2015;15(4):18.
- Umpierrez GE, Smiley D, Jacobs S, Peng L, Temponi A, Mulligan P, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2): 256-261.
- Umpierrez GE, Smiley D, Zisman A, Prieto LM, Palacio A, Ceron M, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30(9):2181-6.
- Brand DA, Peragallo-Dittko V, Fazzari MJ, Islam S, Jacobson AM, Radin MS. Changing to basal-bolus insulin therapy for the inpatient management of hyperglycemia – a natural experiment. Endocr Pract. 2019;25:836-45.
- van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359-67.
- Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180(8):821-7.
- Colunga-Lozano LE, Gonzalez Torres FJ, Delgado-Figueroa N, Gonzalez-Padilla DA, Hernandez AV, Roman Y, et al. Sliding scale insulin for non-critically ill hospitalised adults with diabetes mellitus. Cochrane Database Syst Rev. 2018;11:CD011296.
- Basal-Bolus versus sliding-scale insulin therapy in the acute care hospital setting: a review of comparative clinical effectiveness and cost-effectiveness. CADTH Rapid Response Reports Ottawa (ON), 2017.
- American Diabetes Diabetes care in the hospital. Diabetes Care. 2016;39(Suppl 1):S99-104.
- Schroeder JE, Liebergall M, Raz I, Egleston R, Ben Sussan G, Peyser A, et al. Benefits of a simple glycaemic protocol in an orthopaedic surgery ward: a randomized prospective study. Diabetes Metab Res Rev. 2012;28(1):71-5.
- Colibaseanu DT, Osagiede O, McCoy RG, Spaulding AC, Habermann EB, Naessens JM, et al. Proactive protocol-based management of hyperglycemia and diabetes in colorectal surgery patients. Endocr Pract. 2018;24(12):1073-85.
- Rushakoff RJ, Sullivan MM, Seley JJ, Sadhu A, O’Malley CW, Manchester C, et al. Using a mentoring approach to implement an inpatient glycemic control program in United States hospitals. Healthc (Amst). 2014;2(3):205-10.
- Maynard G, Schnipper JL, Messler J, Ramos P, Kulasa K, Nolan A, et al. Design and implementation of a web-based reporting and benchmarking center for inpatient glucometrics. J Diabetes Sci 2014;8(4):630-40.
- Certification in inpatient diabetes the world wide web: the joint commission; 2019 [Available from: https://www.jointcommission. org/certification/inpatient_diabetes.aspx. Accessed 06 June 2019.
- Goldberg PA, Bozzo JE, Thomas PG, Mesmer MM, Sakharova OV, Radford MJ, et al. “Glucometrics”– assessing the quality of inpatient glucose Diabetes Technol Ther. 2006;8(5):560-9.
- American Diabetes 15. Diabetes care in the hospital: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1): S173-S81.
- Draznin B, Gilden J, Golden SH, Inzucchi SE, investigators P, Baldwin D, et al. Pathways to quality inpatient management of hyperglycemia and diabetes: a call to Diabetes Care. 2013;36(7):1807-14.
- Alamri N, Seley JJ. Evaluation of several electronic glycemic management systems. J Diabetes Sci Technol. 2018;12(1):60-2.
- Ateya MB, Aiyagari R, Moran C, Singer K. Insulin bolus calculator in a pediatric hospital. Safety and user perceptions. Appl Clin Inform. 2017;8(2):529-40.
- Mathioudakis N, Jeun R, Godwin G, Perschke A, Yalamanchi S, Everett E, et al. Development and implementation of a subcutaneous insulin clinical decision support tool for hospitalized patients. J Diabetes Sci Technol. 2019;13(3):522-32.
- Decision support in Epic helps reduce insulin dosing errors by 50% Epic Systems Corporation; November 13, 2017 [Available from: https://www.epic.com/epic/post/decision-support-epic-helps- reduce-insulin-dosing-errors-50.
- Gianchandani R, Umpierrez GE. Inpatient use of computer-guided insulin devices moving into the non-intensive care unit setting. Diabetes Technol Ther. 2015;17(10):673-5.
- Mabrey ME, McFarland R, Young SL, Cooper PL, Chidester P, Rhinehart Effectively identifying the inpatient with hyperglycemia to increase patient care and lower costs. Hosp Pract (1995). 2014;42(2):7–13.
- • Newsom R, Patty C, Camarena E, Sawyer R, McFarland R, Gray T, et al. Safely converting an entire academic medical center from sliding scale to basal bolus insulin via implementation of the eGlycemic management system. J Diabetes Sci Technol. 2018;12(1):53-9 This paper illustrates the steps that can be taken to initiate eGMS in an institution and utility and benefits of implementing electronic glucose management.
- Mumpower A, Parsons T, McFarland RA, Henderson A, editors. Does glycemic control using eGMS reduce readmission rates for hospitalized patients undergoing CABG? Annual Diabetes Technology Meeting, Bethesda, MD, 2017.
- Aloi J, Ullal J, Chidester P, McFarland R. “AUTO study” AUtomatic titration tO target: subcutaneous basal bolus insulin management using eGMS in the non-ICU setting. American Association of Clinical Endocrinologists (AACE) Annual Scientific & Clinical Congress; Austin, TX: Endocr Pract; 2017. p. 1-326.
- Aloi J, Chidester P, Henderson A, Booth R, McFarland R, Mumford A, et al., editors. Improved inpatient hypoglycemia rates with electronic glycemic management system. American Association of Clinical Endocrinologists (AACE) Annual Scientific & Clinical Congress; May 2016; Orlando, FL: Endocrine Practice.
- Juneja R, Golas AA, Carroll J, Nelson D, Abad VJ, Roudebush CP, et al. Safety and effectiveness of a computerized subcutaneous insulin program to treat inpatient hyperglycemia. J Diabetes Sci Technol. 2008;2(3):384-91.
- Tanenberg RJ, Hardee S, Rothermel C, Drake AJ 3rd. Use of a computer-guided glucose management system to improve glycemic control and address national quality measures: a 7-year, retrospective observational study at a tertiary care teaching Endocr Pract. 2017;23(3):331-41.
- Donsa K, Beck P, Holl B, Mader JK, Schaupp L, Plank J, et al. Impact of errors in paper-based and computerized diabetes management with decision support for hospitalized patients with type 2 diabetes. A post-hoc analysis of a before and after study. Int J Med 2016;90:58-67.
- Spat S, Donsa K, Beck P, Holl B, Mader JK, Schaupp L, et al. A mobile computerized decision support system to prevent hypoglycemia in hospitalized patients with type 2 diabetes mellitus. J Diabetes Sci 2017;11(1):20-8.
- Bode B, Harrison C (eds). Time to target using eGMS to manage inpatient subcutaneous insulin basal bolus International Conference on Advanced Technologies & Treatments for Diabetes; February 2017. Diabetes Technology & Therapeutics: Mary Ann Liebert Inc., Publishers.
- Hulkower RD, Pollack RM, Zonszein J. Understanding hypoglycemia in hospitalized patients. Diabetes Manag (Lond). 2014;4(2): 165-76.
- Ullal J, Mabrey M, Henderson A, McFarland R, Booth R, Aloi J. eGlycemic management system provides safe and effective glycemic control for stroke patients requiring subcutaneous insulin in the hospital setting. Annual Diabetes Technology Meeting; Bethesda, MD, 2015.
- Moxey A, Robertson J, Newby D, Hains I, Williamson M, Pearson SA. Computerized clinical decision support for prescribing: provision does not guarantee uptake. J Am Med Inform 2010;17(1):25-33.
- Pearson SA, Moxey A, Robertson J, Hains I, Williamson M, Reeve J, et al. Do computerised clinical decision support systems for prescribing change practice? A systematic review of the literature (1990-2007). BMC Health Serv Res. 2009;9:154.
 Click Image to Enlarge
Click Image to Enlarge