Publication
Journal of Diabetes Science and Technology
Date
January 2018
Authors
Rosalina Newsom,1 Christopher Patty,1 Emma Camarena,1 Regina Sawyer,1 Raymie McFarland,2 Thomas Gray,1 Melanie Mabrey2
ABSTRACT
Objective: Hyperglycemia is common in the inpatient setting and providers frequently rely on sliding scale insulin. This case study reviews the experience of one hospital moving from high utilization of sliding scale to basal bolus insulin therapy.
Method: This Retrospective Quality Improvement Study describes the journey of clinicians at a 580-bed hospital to convert from high usage of SSI to BBI. Hyperglycemic adult patients prescribed insulin, with/without a diagnosis of diabetes, were included.
Results: Data over the first year showed that patients treated with Glucommander (GM) spent more time in the target range of 70-180 mg/dL than patients treated with non-Glucommander (non-GM), with 2,434 fewer hypoglycemic events and 40,589 fewer hyperglycemic events. Prior to implementation of GM, SSI was close to 95%, BBI at 5%. Within the first month of use, 96% usage of BBI was achieved. Reduction of hypoglycemic events (% of BG <70 mg/dL) by 21% with 2.16% non-GM compared to GM at 1.74% and severe hypoglycemia (% of BG <50 mg/dL) by 50% in the ICU 3% non-GM compared to GM at 1.5%. In addition, patients treated with GM had a shorter LOS than patients treated with non-GM by 3.18 days and used 47.4% less point of care tests per patient.
Conclusion: Glycemic management improved with use of eGMS. The conversion from SSI to BBI enhanced overall patient safety, eliminated the time and effort otherwise required when manually titrating insulin and reduced overall cost of care for patients on insulin therapy.
BACKGROUND
Hyperglycemia affects up to 40% of hospitalized patients and is associated with poor outcomes, including increased mortality, morbidity and length of stay.1-7 Hyperglycemia can be related to poorly controlled or unrecognized/undiagnosed diabetes or hyperglycemia of acute illness. Despite the cause of hyperglycemia, numerous professional organizations have recommended use of scheduled basal and nutritional insulin with correction doses (as adjunct to the scheduled insulin) in hospitalized patients with diabetes or in the event of persistent hyperglycemia.8,9 Sliding scale insulin (SSI) remains well ingrained in the hospital culture with use across almost all practice areas despite better blood glucose control, overall outcomes, decreased length of stay and cost savings with scheduled basal bolus insulin therapy.10-12
Changing clinical practice is challenging, particularly when it has been in place for as many years as sliding scale. Numerous publications have demonstrated SSI has no benefits to glycemic control.10-12 A recent meta-analysis demonstrated no benefit in glycemic control with the use of SSI yet higher rates of hyperglycemia.13 Mulla et al14 demonstrated educating providers on and implementation of basal bolus insulin therapy was not sufficient to sustain practice change and proposed integrating technology and repeated education of providers. Hospital management of hyperglycemia with insulin presents many challenges; emerging technologies using commercial computer guided insulin dosing software are being explored in the diabetes field. Several commercially available programs have FDA clearance and have been studied for efficacy and safety for intravenous (IV) insulin guidance in hospitalized patients including Glucommander™ (Glytec, Waltham, MA), EndoTool System™ (MD Scientific LLC, Charlotte, NC), and GlucoStabilizer™ (Medical Decision Network, Charlottesville, VA). Glucommander is the first and only FDA-cleared comprehensive electronic glycemic management system (eGMS) to demonstrate safety and efficacy of computerized software with SubQ insulin in previous published studies.15
eGMSs have been shown to improve glycemic control, help identify previously unrecognized hyperglycemia, prevent hypoglycemia and its associated complications, and reduce the length of stay.16-18
This article describes a quality improvement project implemented in a large California community teaching hospital in the Central Valley, Kaweah Delta Health Care District (KDHCD). The initial aim of the project was to adopt best practice for inpatient management and improve overall glycemic control.
METHODS
A retrospective quality improvement project was completed over a 12-month period from March 16, 2016, to March 17, 2017, to compare results of previous IV and subcutaneous insulin care managed by hospital providers to that of the provider-ordered, nurse-directed, computer-guided Glucommander software, an FDA-cleared insulin dosing decision support composed of IV, transition, subcutaneous (SubQ), and hospital to home (H2H) modules. Glucommander is part of the eGMS integrated with our medical center’s electronic health record. The primary objective was to measure changes in patient safety via reductions in both severe and moderate hypoglycemia versus baseline (patient day and glucose values <40 mg/dL and <70 mg/dL respectively). Secondary objectives were change in hyperglycemia (patient day and glucose values >180 mg/dL), average daily glucose, percentage of blood glucoses values 70-180 mg/dL once prescribed target range reached and overall utilization of basal-bolus versus SSI, number of point of care test per patient, and length of stay (LOS) was collected. LOS was controlled by similar insulin managed patients to control for health system LOS initiatives. This project was reviewed and approved by the KDHCD Institutional Review Board.
Setting
Kaweah Delta Medical Center in Visalia is part of the 581-bed KDHCD in the heart of California and the central San Joaquin Valley. Tulare County is one of California’s poorest regions, is medically underserved, and has experienced multiple rural hospital closures over the past two decades. The academic regional medical center serves over 454,000 patients in Tulare County, which at 13.3% diabetes prevalence ranks first of the 58 California counties. In addition, Tulare County ranks 50th of the 58 in deaths due to diabetes. Some 33% of all inpatients present with a primary or secondary diagnosis of diabetes, and 46% of cardiac surgery patients have diabetes.
Statistical Methodology
The mean with a measure of variability is reported. A sample preliminary test for the equality of variances indicates that the variances of the groups were significantly different. Therefore, a 2-sample t-test was performed that does not assume equal variance. The P value from the t-tests of the observed sample groups determined statistical 95% significance. For all analyses, reported P values are 2-sided, and P values <.05 were considered significant. Mountain States Health Alliance Quality Analysis using QI Macros SPC version 2010.06 performed all statistical analyses.
RESULTS: CLINICAL AND ORGANIZATIONAL
Clinical Results
Among the 4,239 patients (1,479, 34.9% IV & 2,760, 65.1% SubQ patients), 100% of the patient demographic information was collected and shown in Table 1 with BMI the only category statistically different. The eGMS SubQ module managed 69,256 total insulin recommendations shown in Table 2 with 84% of hospital days titrated for basal insulin and 60% of meals with bolus insulin and 60% of meals covered with correction insulin. Doses edited, modified or not administered were reported at 1.1%, so 98.9% of recommendations were administered.
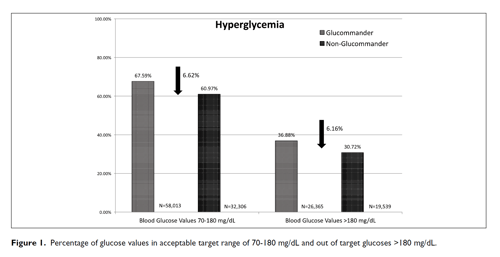
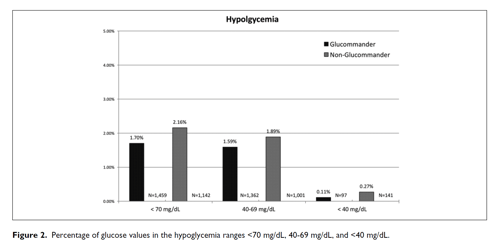
The percentage of severe hypoglycemic events (<40 mg/dL) for GM was 0.11% and 0.27% for non-GM (P <.0001) with mild hypoglycemia (<70 mg/dL) 1.7% for GM and 2.16% for non-GM (P <.001) shown in Table 2. The percentage of severe hypoglycemic patients (<40 mg/dL) for GM was 1.84% and 7.03% for non-GM (P <.0001) with mild hypoglycemia (<70 mg/dL) 20.09% for GM and 30.31% for non-GM (P <.0001). The percentage of BG values between (71-180 mg/dL) for GM was 67.59% and 60.97% for non-GM (P <.0001) and BGs (>180 mg/dL) 30.72% for GM and 36.88% for non-GM (P <.0001) (Figure 1).
Average admission blood glucose for GM was 213.19 ± 106.43 mg/dL and 217.36 ± 127.62 mg/dL for non-GM (P =.65) and decreased for GM at discharge to 167.20 ± 74.15 mg/dL and non-GM to 176.24 ± 61.74 mg/dL (P <.0001). Admission to discharge glucose reduction total 45.99 ± 129.71 for GM compared to 41.12 ± 141.32 mg/dL (P <.0001).
LOS was 5.51 ± 3.19 days for GM and 8.69 ± 4.89 days for non-GM managed patients respectively for a difference of 3.18 days (Figure 2). System variables for LOS improvements was controlled with non-GM insulin managed patients used for control as the comparison was parallel in time with GM managed insulin patients. However, it is important to realize that not all variables with effects on LOS can be practically accounted for, this is a significant limitation of the study.
Table 1. Demographics and Characteristics of Study Patients
| Characteristic | GM | Non-GM | P value |
|---|---|---|---|
| Male Gender | 1664 (52%) | 551 (53%) | NA |
| Age (years) | 63.32 ± 12.45 | 62.83 ± 13.58 | .29 |
| Height (cm) | 166.42 ± 13.29 | 166.47 ± 14.15 | .78 |
| Weight (kg) | 86.51 ± 24.93 | 85.58 ± 26.74 | .23 |
| BMI (kg/m2) | 31.19 ± 5.86 | 30.66. ± 5.89 | .14 |
| Admission BG (mg/dL) | 212.19 ± 101.36 | 217.36 ± 127.62 | .17 |
| GFR (mL/min) | 51.72 ± 12.88 | 49.67 ± 14.65 | .03 |
| Hemoglobin A1C | 8.49 ± 2.0 | 8.76 ± 1.85 | .01 |
| Hospital LOS (days) | 5.51 ± 3.19 | 8.69 ± 4.89 | .0001 |
| Data are mean ± SD or n (%). | |||
Table 2. Glycemic Control and Hypoglycemia in Insulin Patients Managed With Glucommander or Non-Glucommander
| Variable | GM | Non-GM | P value |
|---|---|---|---|
| Number of Patients | 3200 | 1039 | |
| Glucose Control | |||
| Blood Glucose Values | 85,837 | 52,987 | NA |
| Average Blood Glucose Test Per Patient | 26.8 | 50.9 | <.0001 |
| Admission Blood Glucose (mg/dL) | 212.19 ± 101.36 | 217.36 ± 127.62 | .17 |
| Average Hospital Stay Blood Glucose (mg/dL) | 163.07 ± 68.87 | 174.19 ± 74.15 | <.0001 |
| Discharge Blood Glucose (mg/dL) | 167.20 ± 74.15 | 176.24 ± 61.74 | <.0001 |
| Admission to Discharge Blood Glucose Reduction (mg/dL) | 45.99 ± 129.71 | 41.12 ± 141.32 | <.0001 |
| Hyperglycemia | |||
| Blood Glucose Values 70-180 mg/dL | 32,306 (67.59%) | 58,013 (60.97%) | <.0001 |
| Blood Glucose Values >180 mg/dL | 26,365 (30.72%) | 19,539 (36.88%) | <.0001 |
| Hypoglycemia (% patients) | |||
| <70 mg/dL | 643 (20.09%) | 315 (30.31%) | <.0001 |
| 40-69 mg/dL | 627 (19.59%) | 302 (29.07%) | <.0001 |
| <40 mg/dL | 59 (1.84%) | 73 (7.03%) | <.0001 |
| Hypoglycemia (% blood glucose values) | |||
| <70 mg/dL | 1459 (1.7%) | 1142 (2.16%) | <.0001 |
| 40-69 mg/dL | 1362 (1.59%) | 1001 (1.89%) | <.0001 |
| <40 mg/dL | 97 (0.11%) | 141 (0.27%) | <.0001 |
| Data are mean ± SD or n (%). | |||
Organizational Results
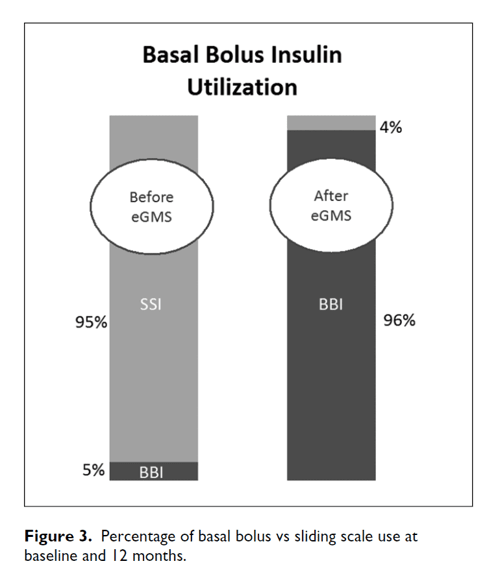
Adopting best practice for inpatient diabetes management and improving overall glycemic control has produced positive organizational changes, improving both the patient experience and provider workflow. For example, baseline insulin use of sliding scale was 95% with 5% basal bolus. At month 1 and sustained through the 12-month study period sliding scale was reduced to 4% and basal bolus improved to 96% (Figure 3).
The number of point of care tests per patient for GM 26.8 and non-GM 50.9 which was a difference of 24.1 tests per patient. The number of point of care test per day for GM was 4.86 and 5.86 for non-GM. Daily titration time per prescriber/per patient was 20.2 ± 27 seconds with GM SubQ compared to 219.7 ± 76 seconds for manual BBI management. Total minutes per shift with a maximum of 9 patients needing insulin management 3.0 (minutes) for GM compared to 33.0 (minutes) for a total reduction of 30 mins per provider per shift for GM SubQ module. Full titration adjustments and edits are displayed in Table 3.
Table 3. Glucommander Insulin Adjustments and Edits
| Variable | n |
|---|---|
| Total Insulin Doses Adjusted | 69,256 |
| Basal Bolus Insulin Therapy, days | 4.39 |
| Basal Insulin Doses Adjusted, % | 0.84 |
| Basal Insulin Doses Adjusted, n | 11,800 |
| Bolus Insulin Doses Adjusted Daily, n | 2.02 |
| Bolus Insulin Doses Adjusted, n | 28,377 |
| Correction Insulin Doses Adjusted Daily, n | 2.07 |
| Correction Insulin Doses Adjusted, n | 29,079 |
| Number of Non-Administered or Modified/Edited Doses, n (%) | 737 (1.1%) |
Improvements in this organization’s management of inpatient diabetes extend well beyond glycemia, utilization, and cost metrics. Our engagement with Glytec over the past 18 months has produced many difficult-to-count but real benefits.
To begin, clinical staff at all levels have an improved awareness of diabetes and its treatment and the implications of disease complications; this awareness is new to our post-GM era. Many clinical staff have participated in comprehensive education on hyperglycemia and diabetes management in the inpatient setting. Although our staff knowledge with respect to diabetes management and insulin therapy has not been formally measured, there is a general perception that the level of knowledge is improved compared to the pre-Glucommander era. We have added routine A1C testing for Glucommander patients that may reveal many undiagnosed cases of diabetes over time. We have eliminated cumbersome IV protocols for cardiac surgery that presented clinical as well as regulatory risk. Our nurses are learning to count carbohydrates and we are having discussion with nutritional services over food quality. Ordering insulin has been simplified, standardized, and is more sustainable.
There is new collaboration between inpatient and outpatient practitioners and departments, and in fact a new masters prepared RN position “measureventionist” has been created. This individual monitors near real-time glycemia measures and can intervene with nursing and medical staff to solve problems and create optimal outcomes. Several new internal publications have been created and sustained, and glycemia data are shared in many more internal venues than in the past.
Supported by Glytec, some of our staff have conducted poster and podium presentations at national and international diabetes conferences. New interprofessional relationships have been developed and new knowledge has been gained.
Discussion and Lessons Learned
Early Implementation
The hospital has long experienced a sense of urgency in improving glycemic control; however, early in the process there was a lack of structured leader-ship and focus. A multidisciplinary, multihospital Diabetes Quality Focus team had been tasked with addressing glycemic management issues. Early focus of the team had led to significant changes in outpatient diabetes care; later the team was restructured with a more comprehensive vision of improving diabetes care across the inpatient/outpatient continuum.
The cardiac surgery service was an exception; there was strong medical staff leadership and motivation to optimize perioperative glycemic control. In fact, the first contacts with Glytec were facilitated by our cardiac surgeons. After on-site presentations and consultation with a wide range of administrative and medical staff specialty leadership, we contracted with Glytec to implement the eGMS for all inpatients.
Clinical Assessment
Shortly after signing the contract, a thorough assessment of current clinical practice related to glycemic control and the care environment was conducted. All disciplines impacting glycemic care participated. The clinical assessment left the project team with a comprehensive listing of current best practice processes in place and a list of gaps to be addressed prior to the eGMS implementation.
Significant gaps were identified in provider and nursing knowledge of insulin pharmacodynamics and kinetics as well as use of basal bolus insulin orders. It was not uncommon for nurses to report holding basal insulin by “nursing judgement” when blood glucoses were in target range for fear of hypoglycemia. Most nurses reported their last education on diabetes care was in nursing school. When presented with a sample clinical scenario of a patient with type 2 diabetes mellitus on metformin, glipizide and sitagliptin as an outpatient who presents to the Emergency Department with cellulitis and blood glucose of 258 mg/dL most providers reported taking a wait-and-see approach to inpatient glycemic control. Varying provider approaches were reported: continuing oral therapy and adding SSI, SSI only, and starting a low dose of basal insulin and SSI. This was regardless of the patient’s nutritional status. The medical and nursing staff demonstrated that opportunities existed to improve understanding and management of the patient requiring insulin therapy. KDHCD had no way of identifying patients in need of glycemic intervention except via direct interaction by the nurse or physician. There were no real-time or daily reports utilized to prompt specific interventions beyond blood glucose monitoring at the bedside.
Recommendations were made to execute a strong educational program to build the foundation of a successful glycemic management program, remove orders for SSI from the electronic health record’s provider order entry, and implement order sets for IV and transition, as well as SubQ Glucommander with weight based insulin dosing. The project started in September 2015 with technical integrations and clinical practice change meetings. Training in use of the eGMS was conducted in February 2016 and the first patients were started on the eGMS March 19, 2016.
Implementation
An interprofessional Glucommander steering committee was established under the codirection of the director of nursing practice and the medical director of performance improvement. This committee was composed of staff from senior administration, nursing, medical staff, pharmacy, clinical education, information systems services, nutritional services, clinical laboratory, clinical engineering, and marketing.
Aside from medical and nursing staff education, there were several foundational tasks needing completion before go-live could be considered. Historical (baseline) point-of-care (POC) glucose results were transmitted, and an interface between the hospital EMR and Glucommander software was established and tested. A significant previously planned change involved the implementation of wireless POC glucometers to shorten testing cycle time and enable faster upload of glycemia results to Glucommander. Access to clinical performance reports (Glucommander/GlucoMetrics) was established.
At time of “go-live,” a “command center” was created and staffed around-the clock for the first week by an advanced practice nurse and clinical services coordinators (nurses and CDEs) from Glytec. A phased approach was taken in the initiation of Glucommander therapy; patients already hospitalized before go-live were maintained on SSI therapy until discharge. It was felt that this approach would facilitate early problem-solving and “learning curve” issues for staff. Pharmacists reviewed all patients on any insulin therapy daily and worked with the Glytec team to initiate insulin therapy where indicated and convert those on sliding scale to GM. Within the week, almost all insulin therapy was managed using Glucommander, a few longer-stay patients were converted from SSI therapy.
Daily afternoon debriefings were held in the command center and issues and resolutions were aggregated into a “FAQ” that was circulated daily among clinical staff. An advanced practice RN staff was assigned to a “roving” role and visited staff caring for Glucommander patients several times daily.
Change Management and Success Strategies
Change is hard, and even positive change requires evaluating staff resistance and implementing strategic leadership solutions. Significant concern came from medical staff, and it should be noted that many physicians had not utilized the Glucommander education tools prior to ordering therapy for the first time. Representative comments indicating concern included “sliding scale is easier – it’s what patients use at home,” “I don’t have the time to ask about details of home management,” GM is not appropriate for some patients,” “nurses call me less when I order SSI,” “I already know how to order insulin,” “we didn’t agree to this,” “hypoglycemia is worse now,” and “block GM if you can.”
A few high-leverage strategies produced the most significant improvements in reducing provider concerns. For example, through analysis of hypoglycemia data, we “discovered” (before we read the user’s manual) that patients with an A1C less than 6.5 may not require GM. After six months of experience, hypoglycemia in these patients had been sharply reduced, especially in the “normal” A1C range patients. Another successful strategy was the creation of a daily report listing provider orders for non-Glucommander insulin orders. The medical director of PI worked with individual (resistant) prescribers, and much of the resistance was overcome. Last, APRNs, experienced in critical care, quickly became knowledgeable resources and guided many physicians through their first few Glucommander patients.
Lessons Learned
- Know the scope of your community needs, challenges, and resources.
- Assume knowledge deficits exist for providers, nurses and leaders, and correct them through education before implementation.
- Measure pre- and post-implementation levels of diabetes and insulin management knowledge using a valid and reliable tool.
- Old habits are hard to break.
- Strong executive leadership is key to success.
- Strong understanding of baseline metrics is vital.
- A project manager is critical to maintaining focus.
- Essential to have a solid plan and continuous communication.
- Be courageous, take the leap!
Recommendations for others considering a project of this type include requiring all medical and nursing staff to attend updated inpatient diabetes and hyperglycemia education before go-live. Having a strong knowledge base to build upon may have made our project move more smoothly in the early implementation. In addition, education of end users on the use of the new insulin order sets and specifically providers understanding appropriate ordering of GM would also be helpful.
While SSI may not be dead as Childs suggested in 2003,19 we have moved the needle tremendously in the right direction and are providing best practice for our patients.
Abbreviations
BBI, basal bolus insulin; eGMS, electronic glycemic management system; EMR, electronic medical record; GM, Glucommander; H2H, hospital to home; IV, intravenous; KDHCD, Kaweah Delta Health Care District; LOS, length of stay; non-GM, non-Glucommander; POC, point of care; SSI, sliding scale insulin; SubQ, subcutaneous.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MM and RM are full-time employees of Glytec. No other disclosures.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
AFFILIATIONS
- Kaweah Delta Health Care District, Visalia, CA, USA
- Glytec, Waltham, MA, USA
REFERENCES
- Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978-982.
- Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67(2):352-360; discussion 360-352.
- Malmberg K, Norhammar A, Wedel H, Ryden L. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation. 1999;99(20):2626-2632.
- McAlister FA, Majumdar SR, Blitz S, Rowe BH, Romney J, Marrie TJ. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community-acquired pneumonia. Diabetes Care. 2005;28(4):810-815.
- Pomposelli JJ, Baxter JK III, Babineau TJ, et al. Early post-operative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr. 1998;22(2):77-81.
- Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30(9):2181-2186.
- Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256-261.
- Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353-369.
- Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16-38.
- Akhtar ST, Mahmood K, Naqvi IH, Vaswani AS. Inpatient management of type 2 diabetes mellitus: does choice of insulin regimen really matter? Pak J Med Sci. 2014;30(4):895-898.
- Gomez Cuervo C, Sanchez Morla A, Perez-Jacoiste Asin MA, Bisbal Pardo O, Perez Ordono L, Vila Santos J. Effective adverse event reduction with bolus-basal versus sliding scale insulin therapy in patients with diabetes during conventional hospitalization: systematic review and meta-analysis. Endocrinol Nutr. 2016;63(4):145-156.
- Phillips VL, Byrd AL, Adeel S, Peng L, Smiley DD, Umpierrez GE. A comparison of inpatient cost per day in general surgery patients with type 2 diabetes treated with basal-bolus versus sliding scale insulin regimens. Pharmacoecon Open. 2017;1(2):109-115.
- Lee YY, Lin YM, Leu WJ, et al. Sliding-scale insulin used for blood glucose control: a meta-analysis of randomized con-trolled trials. Metabolism. 2015;64(9):1183-1192.
- Mulla CM, Lieb DC, McFarland R, Aloi JA. Tides of change: improving glucometrics in a large multihospital health care system. J Diabetes Sci Technol. 2015;9(3):602-608.
- Aloi J, Bode B, Ulla J, et al. Comparison of an electronic glycemic management system versus provider managed subcutaneous basal bolus insulin therapy in the hospital setting. J Diabetes Sci Technol. 2017;11(1):12-16.
- Umpierrez G, Cardona S, Pasquel F, et al. Randomized con-trolled trial of intensive versus conservative glucose control in patients undergoing coronary artery bypass graft surgery: GLUCO-CABG trial. Diabetes Care. 2015;38(9):1665-1672.
- Aloi J, Mulla C, Ullal J, Lieb D. Improvement in inpatient glycemic care: pathways to quality. Curr Diab Rep. 2015;15(4):18.
- Newton C, Young S. Financial implications of glycemic control: results of an inpatient diabetes management program. Endocr Pract. 2006;12(suppl 3):43-48.
- Childs BP. Death to the sliding scale! Diabetes Spectr. 2003;16(2):68-69.