Evaluating the Impact of Glucommander on Improvement in Time-in-Range (TIR) in Type 2 Diabetes using Continuous Glucose Monitoring
Presentation
ADA Virtual 80th Scientific Sessions
Date
June 13, 2020
Authors
Bruce Bode, MD, FACE, John Clarke, RN, CDES
PURPOSE
An IRB-approved proof-of-concept single-center prospective study to evaluate the safety and efficacy of utilizing Glucommander outpatient insulin dosing software to assist providers in titrating MDI basal bolus insulin doses, using glucose data from Abbott Freestyle Libre 14-day glucose monitoring system.
SETTING
Single Center: Atlanta Diabetes Associates
HARDWARE
Abbot Freestyle Libre 14-day Glucose Monitoring System and cell phone for using Abbott LibreLink app
SOFTWARE
Glytec Glucommander Outpatient insulin dosing software (Cloud)
Abbott Freestyle LibreLink client-facing app & LibreView provider-facing portal (Cloud)
DATA
- Prospective data from 25 adult patients
- Enrollment Criteria: Age=(18 to 80); Type 2 Diabetes; A1C > 8.0%. Must have an iPhone or Android phone capable of running the LibreLink app..
- Exclusion Criteria: eGFR<30; hemoglobinopathy; steroid use; pregnancy
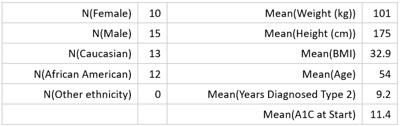
DEMOGRAPHICS
METHODS
- Visit 1: Training by a nurse educator about MDI basal-bolus insulin administration, meal planning, and the use of a glucose sensor. Collection of baseline data begins.
- Visit 2: Start of Glucommander program.
- Visits 3 through 6: The patient’s CGM data from each newly completed week were used by the software to provide new insulin dose titrations.
- Visit 7: Collect final sensor data from the 4th adjustment.
ANALYSIS
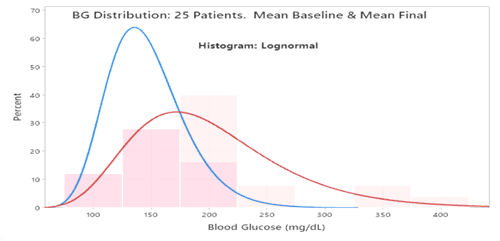
Statistical comparisons were conducted between the pooled baseline data vs. pooled final data. The mean of the patients’ mean BGs was also compared between baseline & final. Specifications are in the references 1,2,3,4
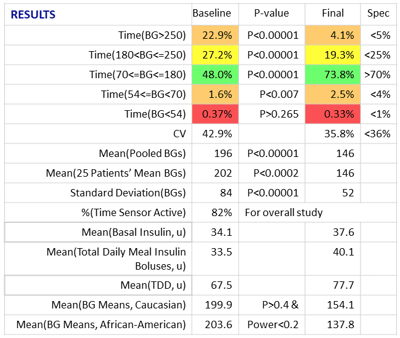
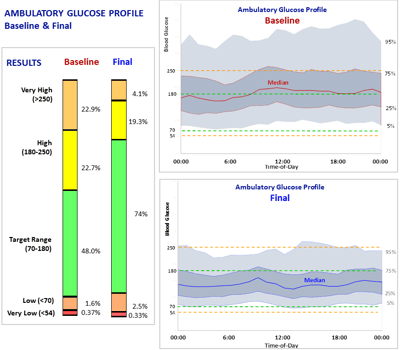
RESULTS
CONCLUSIONS
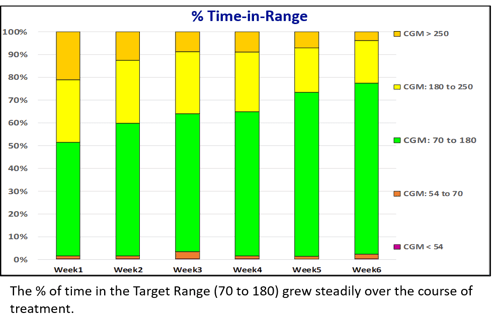
Glucommander software and CGM data were used to calculate safe and effective dose adjustments, to optimize the efficacy of injected basal bolus insulin. Time in range improved from 48.0% to 73.8% while staying well within AGP-acceptable norms for avoiding hypoglycemia. An RN, CDES accomplished these results with four consecutive weekly titrations, each requiring only a visual review of the data and a touch of a button, without intervention from the ordering licensed provider.
This approach delivers significantly improved outcomes for patients on basal bolus insulin with multiple daily injections, while optimizing the use of clinical resources. The combination of Glucommander software and CGM data can continually optimize insulin doses and improve outcomes while relieving the burden on patients and providers.
References
- Section 6. Glycemic Targets: Standards of Medical Care in Diabetesd2020; Diabetes Care 2020;43(Suppl. 1):S66–S76 .
- Battelino T, Danne T, Bergenstal R, Amiel S, Beck R, Biester T, Bosi E, Buckingham B, Cefalu W, Close K, Cobelli C, Dassau E, DeVries H, Donaghue K, Dovc K, Doyle F, Garg S, Grunberger G, Heller S, Heinemann L, Hirsch I, Hovorka R, Jia W, Kordonouri O, Kovatchev B, Kowalski A, Laffel L, Levine B, Mayorov A, Mathieu C, Murphy H,Nimri R, Nørgaard K, Parkin C, Renard E, Rodbard D, Saboo B, Schatz D, Stoner K, Urakami T, Weinzimer S, and Phillip M; Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations from the International Consensus on Time in Range; Diabetes Care 2019;42:1593–1603 .
- Beck R, Bergenstal R, Riddlesworth T, Kollman C, Li Z, Brown A, and Close K; Validation of Time in Range as an Outcome Measure for Diabetes Clinical Trials; Diabetes Care 2019;42:400-405.
- Bergenstal R, Ahmann A, Timothy Bailey T, Bissen J, Buckingham B, Deeb L, Dolin R, Garg S, Goland R, Hirsch I, Klonoff D, Kruger D, Matfin G, Mazze R, Olson B, Parkin C, Peters A, Powers M, Rodriguez H, Southerland P, Strock E, Tamborlane W, and Wesley D; Recommendations for Standardizing Glucose Reporting and Analysis to Optimize Clinical Decision Making in Diabetes: The Ambulatory Glucose Profile; Journal of Diabetes Science and Technology Vol 7, Issue 2, March 2013.
ECO #0852-A