Evaluating the Impact of eGMS Glucommander on Length of Stay, Hypoglycemia and Glucose Control in a Regional Medical Center
Presentation
Annual Diabetes Technology Meeting
Date
November 2018
Authors
LaTivia Carr, Cheryl Rogers, Daniel Ryan, Raymie McFarland, Nives Bernardi, Kelly Frey, Trisha Haines
BACKGROUND
Healthcare organizations face numerous challenges when implementing glycemic management improvement initiatives. This study examines differences in outcomes between patients whose insulin titrations were managed using the Glytec® eGlycemic Management System® (eGMS®) Glucommander™ software and patients whose insulin titrations were managed using standard (paper) protocols, with a focus on COPD, CHF and DKA populations.
METHODS
The aim of this retrospective quality improvement study was to compare the clinical and financial outcomes of eGMS® Glucommander™ (GM) to standard (paper) protocols (SP) in the critical care units of a 335-bed regional medical center. Twelve months of data, from November 1, 2016 through October 31, 2017, was collected and analyzed on patients requiring glucose management with intravenous and/or subcutaneous insulin.
| Patient Characteristics | GM | SP | p value |
|---|---|---|---|
| Number of Patients | 174 | 208 | |
| Age (years) | 62.44 | 63.28 | >0.05 |
| Gender – Female | 47% | 48% | |
| Gender – Male | 53% | 52% | |
| A1C (%) | 8.14 | 8.73 | <0.05 |
| BMI | 31.18 | 31.08 | >0.05 |
| Starting BG (mg/dL) | 300.32 | 263.85 | <0.05 |
RESULTS
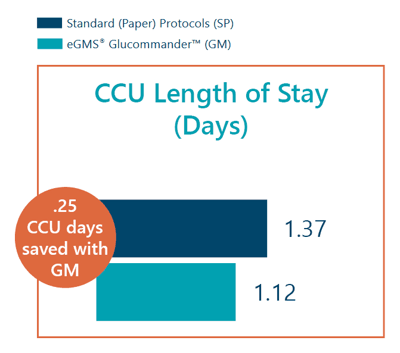
- CCU length of stay index was 1.12 days with GM vs 1.37 days with SP.
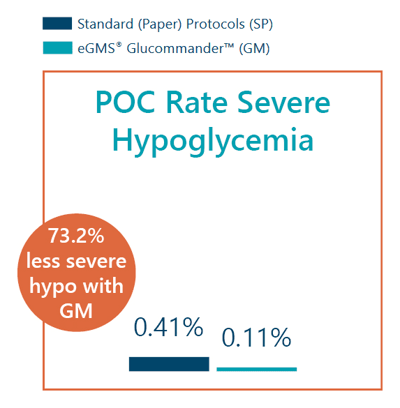
- Point-of-care rate of severe hypoglycemia was 0.11% with GM vs 0.41% with SP.
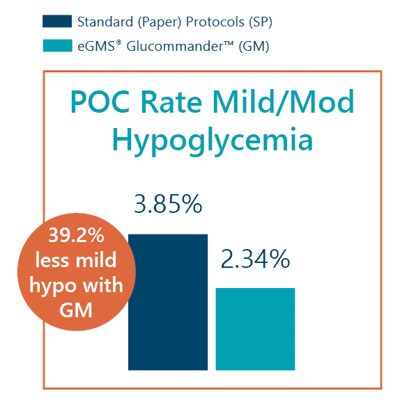
- Point-of-care rate of mild-to-moderate hypoglycemia was 2.34% with GM vs 3.85% with SP.
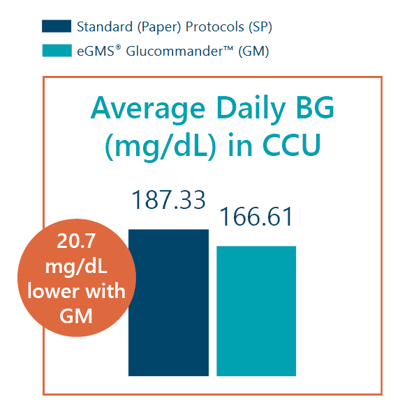
- Average daily blood glucose in the CCU was 166.61 mg/dL with GM vs 187.33 mg/dL with SP.
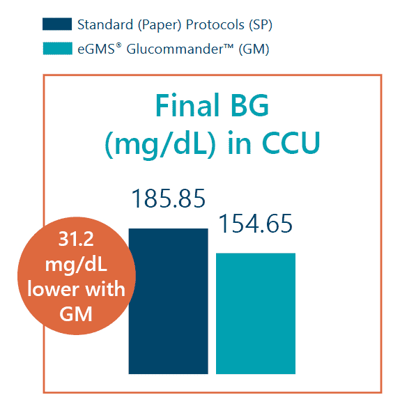
- Average final blood glucose in the CCU was 154.65 mg/dL with GM vs 185.85 mg/dL with SP.
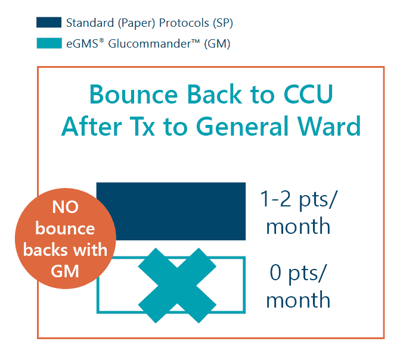
- Bounce-back rate for transfers from the CCU to general wards was zero patients per month with GM over the study period vs 1-2 patients per month with SP over the 12 months prior.
CONCLUSIONS
eGMS® Glucommander™ provided a decreased CCU length of stay of .25 days compared to standard protocols. Patients treated using eGMS® Glucommander™ experienced less overall hypoglycemia than patients treated using standard protocols, and eGMS® Glucommander™ was more effective at reaching ADA targets for average daily blood glucose and final blood glucose.