Blood Glucose Control Using a Computer-Guided Glucose Management System in Allogeneic Hematopoietic Cell Transplant Recipients
Publication
Bone Marrow Transplant
Date
April 2016
AUTHORS
C Espina,1 I Jenkins,2 L Taylor,1,2 R Farah,3 E Cho,1,2 J Epworth,1 K Coleman,1,2 J Pinelli,1,2 S Mentzer,1,2 L Jarrett,2 T Gooley,2 P O’Donnell,1,2,4 I Hirsch,1 M Bar,1,2
ABSTRACT
Allogeneic hematopoietic cell transplantation (HCT) is a potentially curative treatment for patients with hematological malignancies. However, is associated with substantial rates of morbidity and mortality. We and others have shown that malglycemia is associated with adverse transplant outcome. Therefore, improving glycemic control may improve transplant outcome. In this prospective study we evaluated the feasibility of using Glucommander (a Computer-Guided Glucose Management System; CGGM) in order to achieve improved glucose control in hospitalized HCT patients. Nineteen adult patients contributed 21 separate instances on CGGM. Patients were on CGGM for a median of 43 h. Median initial blood glucose (BG) on CGGM was 244 mg/dL, and patients on 20 study instances reached the study BG target of 100-140 mg/dL after a median of 6 h. After BG reached the target range, the median average BG level per patient was 124 mg/dL. Six patients had a total of 10 events of BG <70 mg/dL (0.9% of BG measurements), and no patients experienced BG level <40 mg/dL. The total estimated duration of BG <70 mg/dL was 3h (0.2% of the total CGGM time). In conclusion, our study demonstrates that stringent BG control in HCT patients using CGGM is feasible.
Bone Marrow Transplantation (2016) 51, 973–979; doi:10.1038/bmt.2016.78; published online 4 April 2016.
INTRODUCTION
Hyperglycemia is associated with an increased risk of death among patients with both chronic and acute illnesses.1-3 Recently, efforts have been made to control hyperglycemia during hospitalization, but results from clinical trials and retrospective analyses have demonstrated inconsistent outcomes associated with intensive insulin therapy.4–9,10-12 There is also evidence that severe hypoglycemia (glucose <40 mg/dL) in hospitalized patients may have a detrimental effect on outcomes, including cardiac arrest, seizures, hypoglycemia-induced coma and mortality,5,13-17 thus limiting the efforts for meticulous glucose control in the hospital. In addition, recent evidence also suggests that glucose variability can be detrimental and increase mortality risk in hospitalized patients.18-21
Allogeneic hematopoietic cell transplantation (HCT) is the only potential curative treatment for patients with high-risk hematological malignancies. However, HCT continues to be associated with substantial rates of non-relapse mortality (NRM) (3-year NRM of 20-30%).22,23 Our team has previously shown that malglycemia (hyperglycemia, hypoglycemia or increased glycemic variability) was associated with increased day-200 NRM, overall mortality and infection rate. Our earlier study demonstrated that glucose values above 200 mg/dL were associated with twofold or more increase in NRM, compared with BG values between 101 and 150 mg/dL.24 Similarly, Fuji et al.25 demonstrated an association between hyperglycemia and increased risk of organ dysfunction, grade II-IV acute GvHD and NRM in adult patients treated by myeloablative allogeneic HCT, and Gebremedhin et al.26 demonstrated that severe hyperglycemia immediately after allogeneic HCT was predictive of acute GvHD. There is, therefore, interest in improving glycemic control in the hope of improving transplant outcome. Fuji et al. previously conducted an intensive glucose control study after HCT. Although the result was feasible, glucose control in that study was still unsatisfactory.27 Computer-Guided Glucose Management (CGGM) may be a mechanism through which intensive glucose control can be attained. In this prospective study we evaluated the feasibility of using Glucommander 1.0 (an FDA (Food and Drug Administration)-cleared CGGM) in order to achieve improved glucose control in hospitalized HCT patients. Based on the findings of our prior study24 the primary objective of this study was to examine the ability of CGGM algorithm to control glucose level within target range of 100-140 mg/dL.
SUBJECTS AND METHODS
Patients
Patients 18 years of age and older who underwent allogeneic HCT at the FHCRC (Fred Hutchinson Cancer Research Center), Seattle, and required insulin therapy due to known history of type 2 diabetes, two episodes of blood glucose (BG) level above 180 mg/dL or one BG level above 250 mg/dL were eligible for the study. Exclusion criteria for participation were critically ill patients (Intensive Care Unit status), terminally ill patients, Eastern Cooperative Oncology Group >3 and diagnosis of type 1 diabetes mellitus. The study received approval by the FHCRC Institutional Review Board.
Nineteen adult patients were treated on the study. Two of those patients were treated on the study during two separate hospitalizations, resulting in a total of 21 separate instances on CGGM.
Table 1. Patient Characteristics
| Characteristic | N (%) or median (range) |
|---|---|
| Number of patients | 19 |
| Number of study instances | 21 |
| Male patientsa | 13 (68%) |
| Male instances | 15 (71%) |
| Age at enrollment (years) | 52 (22-73) |
| Days from hematopoietic cell transplantation to enrollment | 67 (8–479) |
| BMI (kg/m2) | 29 (19–41) |
| Diagnosisa | |
| AML | 8 (42%) |
| ALL | 4 (21%) |
| MDS | 3 (16%) |
| NHL | 2 (11%) |
| CML | 1 (5%) |
| HL | 1 (5%) |
| Donor typea | |
| Related allo | 7 (37%) |
| URD | 12 (63%) |
| Conditioninga | |
| Ablative | 14 (74%) |
| Nonablative | 5 (26%) |
| Prior T2DM | 3 (14%) |
| GvHD prior to enrollment | 18 (86%) |
| Steroids (prednisone) | 19 (90%) |
| Daily prednisone dose at enrollment (mg) | 140 (10–200) |
| Total parenteral nutrition | 10 (48%) |
Abbreviations: BMI = body mass index; HL = Hodgkin lymphoma; MDS = myelodysplastic syndrome; NHL = non Hodgkin lymphoma; T2DM = type 2 diabetes; URD = unrelated donor.
aIndicates percent calculated out of 19 patients; otherwise percent calculated out of 21 CGGM instances.
BG monitoring and insulin treatment
BG measurements were obtained by bedside capillary glucose finger stick or venous whole blood, using Roche Accu-Chek Inform glucose meter. Insulin was given as continuous IV infusion. BG levels were entered manually into the Glucommander Software, which calculated the insulin dose for the next period, and calculated the time for the next BG measurement. Changes to the insulin infusion doses were made manually by the nursing staff, according to the Glucommander software recommendations.
Glucommander software
Glucommander (Glytec, Waltham, MA, USA) is an FDA-cleared CGGM system.28 Using a physician-selected glucose target range and a weightbased multiplier, the computer-based algorithm calculates and recommends an insulin infusion rate and interval to next BG measurement. To initiate the system a desired BG target, patient’s weight and BG value are manually entered by a health-care provider, and the initial insulin infusion rate and the time for the next BG measurement are calculated by the software. The interval for BG measurements is between 20 and 120 min based on the rate of glucose level change. The interval of BG checks recommended by Glucommander is consistent with the standard of care for insulin infusion, typically every 60 min. Interval is extended to every 120 min if patient is within target range for a specified time, or shortened to every 20 min based on the rate of change of BG level. The system continues recommending the IV insulin infusion rate until discontinued by the health-care provider.
Statistical analysis
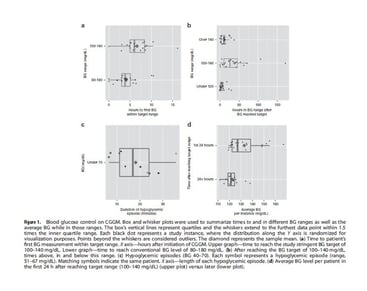
Box and whisker plots were used to summarize times to and in different BG ranges as well as the average BG while in those ranges. The box’s vertical lines represent quartiles and the whiskers extend to the furthest data point within 1.5 times the inner quartile range. Times to the specified BG range were calculated as the number of hours from initiation of CGGM to the first measurement within that range. Times in the BG ranges were calculated by assuming a linear relationship between any two values and imputing the time a threshold was crossed. Two of the 19 study participants contributed two separate instances on CGGM, their data were treated as independent for these descriptive analyses, resulting in 21 separate study instances. Simple linear regression and LOESS smoothing were used to highlight trends in the data. Analyses were completed with the statistical computing language R v3.2.229 and the ggplot2 graphics package v1.01.30
RESULTS
Patient characteristics
Nineteen adult patients (13 male), contributed 21 separate instances on CGGM at a median of 69 days (range, 9-481 days) after allogeneic HCT. Median age at study enrollment was 52 years (range, 22-73 years). Patients on 19 of the 21 study instances were on systemic steroids for treatment of GvHD (n=18) or diffuse alveolar hemorrhage (n=1), with median daily prednisone dose of 140 mg (range, 10-200 mg). Patients on six study instances had documented active infection. Total parental nutrition was given to patients on 10 out of 21 study instances. Three patients had a prior history of type 2 diabetes. Characteristics of patients are presented in Table 1.
Patients remained on CGGM for a median of 43h (range, 10-195 h) for a total of 1272 h. Patients were removed from CGGM due to: transition from IV insulin to SC insulin (n=9), patient request (n=5), discharge (n=4), other (n=3).
Glucose control
Median BG at initiation of CGGM, defined as the first BG reading while on CGGM, was 244 mg/dL (range, 124-421). Patients on 20 of the 21 study instances reached the study BG target of 100-140 mg/dL after a median of 6h (range, 0-16.2 h) (Figure 1a). There was one instance where the initial BG on CGGM was within 100-140 mg/dL range, as the patient was on standard insulin therapy prior to initiation of CGGM. Thus, for this patient, it took zero hours to reach the target range. The one patient who did not reach the study BG target (study instance no. 21, Figure 4) entered the CGGM with BG level of 421 mg/dL, reached a BG level of 157 mg/dL, and was removed from study after 11.5h due to patient request. After BG reached the study target range (100-140 mg/dL), the median average BG level per patient was 124 mg/dL (range of 118-167). All patients reached a conventional BG range of 80-180 mg/dL at a median time of 3.8h (range, 0-10.5 h) (Figure 1a).
Patients had wide variability in glucose level before and after CGGM, while maintaining targeted BG level (100-140 mg/dL) in 61% of the time while on CGGM (Figure 2). For comparison, patients were within target range only 0.58% of the time during the last 24h prior to initiation of CGGM and 20.95% of the time in the 24h after completion of CGGM.
After reaching the target range, patients spent a median of 3.8h under 100 mg/dL and 10.2h over 140 mg/dL, compared with 26.2h within the 100-140 mg/dL range (Figure 1b). Stringent glucose control (100-140 mg/dL) was achieved with only 10 documented hypoglycemic episodes (BG <70 mg/dL; range, 51-67 mg/dL) (0.9% of BG measurements), experienced by six patients (Figure 1c). All six patients who developed hypoglycemia had GvHD and were treated with systemic glucocorticoids at an average dose of 105 mg/day and three patients also had active infections. With the limitation of timing of bedside BG measurements by nursing staff, the estimated median duration of hypoglycemic episodes was 17 min (range, 6-36 min). The total estimated duration of BG <70 mg/dL for the entire study cohort was 3h (0.2% of the total time patients were monitored on CGGM) (Figure 1c). No severe hypoglycemic episodes (BG <40 mg/dL) were detected.
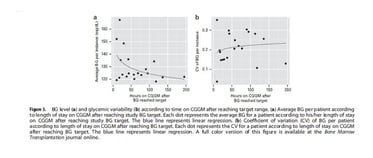
Patients on 13 of the 21 study instances remained on CGGM for >24h after reaching the target range. Median BG per patient was 127 mg/dL (range, 119-167 mg/dL) in the first 24h after reaching the target, and was 121 mg/dL (range, 112-138 mg/dL) thereafter (Figure 1d). Our data suggest a trend for better BG control the longer the patient remained on CGGM (Figure 3a), but with no difference in BG variability (Figure 3b).
Figure 4 demonstrates BG levels of all patients while on CGGM. Insulin doses required to maintain tight glucose control while on CGGM were variable (between 0 and 59.6 u/h), but were higher than the insulin doses used in the 24h before or after CGGM. Table 2 summarizes the range of insulin doses for all patients while on CGGM and in the 24h before and after CGGM.
Table 1. Insulin doses before, during and after CGGM
| Study participant no. | GvHD status at enrollment | Daily prednisone dose (mg) | Infection status | Total Insulin dose during the last 24 h before CGGM |
Range insulin dose during CGGM | Total Insulin dose during the first 24 h after CGGM |
|---|---|---|---|---|---|---|
| 1 | Active GvHD | 100 | CMV reactivation | 0 | 4.1-28 u/h | Glargine 20u, Lispro 8u |
| 2 | Active GvHD | 50 | CMV reactivation E. coli bacteremia | Lispro 6u | 1.6-5.9 u/h | Lispro 5u |
| 3 | Active GvHD | 90 | CMV reactivation | Lispro 91u | 5.1-59.6 u/h | Lispro 75u, Glargine 25u |
| 4 | Active GvHD | 176 | None | Lispro 51u | 1.4-–5.1 u/h | Glargine 30u, Lispro 17u |
| 5 | Active GvHD | 90 | None | Lispro 10u | 1-5.6 u/h | None |
| 6 | Active GvHD | 60 | None | Lispro 22u | 2.4-23.8 u/h | NPH 32u, Lispro 24 |
| 7 | Active GvHD | 85 | CMV reactivation E. coli bacteremia | Lispro 24u | 1-10.4 u/h | Glargine 50u, Lispro 24u |
| 8 | No GvHD | 0 | Neutropenic fever | Lispro 5u | 1.5-18.5 u/h | Lispro 5u |
| 9 | Active GvHD | 200 | Staph coagulase-negative bacteremia | Glargine 15u, Lispro 5u | 1.4-12.3 u/h | Glargine 24u, Lispro 49u |
| 10 | Active GvHD | 140 | Rhinovirus | Lispro 71u | 1.5-16.6 u/h | Glargine 56u, NPH 12u, Lispro 91u |
| 11 | Active GvHD | 0 | Neoutropenic fever | Lispro 13u | 3-19. 2 u/h | Glargine 50u, Lispro 24u |
| 12 | Active GvHD | 150 | CMV reactivation | Lispro 18u | 1.8-8.8 u/h | Glargine 40u, Lispro 2u |
| 13 | Active GvHD | 65 | None | Lispro 14u | 1.8-9.5 u/h | Regular 60u |
| 14 | Active GvHD | 10 | Sepsis picture, no source of infection identified | Regular 70.4u | 0.3-22. 8u/h | Glargine 20u, Lispro 44u |
| 16 | Active GvHD | 170 | None | Lispro 20u | 0-25.2 u/h | Glargine 48u, Lispro 38u |
| 17 | Active GvHD | 150 | None | Lispro 21u | 0.4-6.6 u/h | NPH 30u, Lispro 22u |
| 18 | Active GvHD | 160 | None | Lispro 10u | 0-6.8 u/h | Lispro 14u |
| 19 | Active GvHD | 160 | None | Lispro 16u | 0.6-20.6 u/h | NPH 26u, Lispro 35u |
| 20 | Active GvHD | 75 | CMV enteritis | Glargine 20u, Lispro 14u | 2-7.2 u/h | NPH 30u, Lispro 20u |
| 21 | No GvHD (steroids for DAH) | 152 | Aspergillus pneumonia | Glargine 20u, Lispro 21u | 7.7-23.1 u/h | NPH 60u, Lispro 30u |
| 22 | Active GvHD | 180 | CMV reactivation, Aspergillus pneumonia | Glargine 15u, Lispro 16u | 0-11.6 u/h | Glargine 83u, regular 10u, Lispro 38u |
Abbreviations: CGGM = Computer-Guided Glucose Management System; DAH = diffuse alveolar hemorrhage.
DISCUSSION
Several studies have evaluated the effect of BG levels on hospitalized patients with emerging evidence that not only hyperglycemia, but also hypoglycemia and variable glucose level may have a negative effect on outcome.1,2,5,8,14-16,18-20,31-36 Our team and others have previously demonstrated the association between malglycemia (hyperglycemia, hypoglycemia and increased glycemic variability) and increased NRM and infections in HCT patients.24-26 In addition, adverse clinical consequences of hyperglycemia from total parenteral nutrition exposure during HCT were reported.37 These findings raised the hypothesis that stringent glycemic control with prevention of hypoglycemia and glucose variability may improve transplant outcome. However, as demonstrated by the NICE SUGAR study, intensive glucose control may increase the risk of severe hypoglycemia.35 Thus, a novel strategy is required to obtain glucose level within a stringent range while minimizing hypoglycemia. Using Glucommander it was recently shown that for patients without diabetes, complications during coronary artery grafting were less, maintaining glucose level between 100 and 140 mg/dL versus level of 141-180 mg/dL.12 As the first step to prospectively evaluate the effect of this desired degree of glucose control in the HCT setting, we evaluated the feasibility of obtaining near normoglycemia in HCT patients using a CGGM. Despite the small number of patients enrolled on the study, we demonstrated that a narrow range of glucose levels between 100 and 140 mg/dL was attained 61% of the time while on CGGM, with only 10 episodes (0.9% of BG measurements and 0.2% of total time on study) of BG lower than 70 mg/dL and with no episodes of BG lower than 40 mg/dL. This rate is significantly lower compared with other protocols. For example, in the NICE SUGAR study 6.9% of patients in the intensive therapy group had severe hypoglycemia defined as a glucose level below 40 mg/dL.38 Our data, in a more difficult population, are comparable to other CGGM data, in which, among over 5000 insulin runs (over 120 000 h) 0.6% of values were found to be <50 mg/dL.28 Hypoglycemia will always remain a limiting factor of insulin therapy, but the frequency of hypoglycemia in our study is minimal as we documented no levels below 50 mg/dL and only 0.9% below 70 mg/dL.
GvHD produces a massive inflammatory response,39 which may lead to insulin resistance.40 Treatment with glucocorticoids, while effective in reducing inflammatory activation, will result in more insulin resistance and for many, hyperglycemia, as demonstrated by Pidala et al.41 There are further theoretical concerns that the combination of hyperglycemia and hyperinsulinemia (either endogenous or exogenous) may be maladaptive as this scenario has been shown to result in additional inflammation.42 On the other hand, when enough insulin is provided to control glycemia, inflammation is actually suppressed.43 As recently reviewed by Fuji et al. hyperglycemia causes not only impaired immune function and elevation of proinflammatory cytokines, but also causes problems with other tissues, such as endothelial dysfunction, catabolism of muscle and fat and procoagulation, all of which may be relevant in patients after allogeneic HSCT.44 Given our original observations of increased NRM and mortality associated with malglycemia,24 we hypothesized that providing enough IV insulin to meticulously control glucose levels during GvHD could add to the anti-inflammatory stimulus of the glucocorticoids and impact HCT outcomes. The primary objective of our study was to evaluate if the degree of glycemic control required in this situation was possible, and the results suggest it is indeed possible, although inflammatory cytokines were not measured.
A limitation of our study was the need for frequent (approximately once every hour) BG measurements to be entered to the CGGM software to calculate the recommended insulin dose. The frequent BG measurements by finger stick caused inconvenience to patients and nursing staff, but were essential to adjust the insulin dosing in order to maintain glucose levels within the target range. Furthermore, current standard IV insulin protocols also require hourly BG testing.45,46 While continuous glucose monitoring, either SC or intravascular are not current options in the inpatient setting, if these tools ever become available the ability to control glycemia should become less burdensome.47-51 Additional limitations of our study are the small cohort and the lack of a control arm. However this is a feasibility study, which we hope will set the stage for a larger randomized trial.
In conclusion, our study demonstrates that despite high doses of steroids, total parenteral nutrition, and unpredictable oral intake, stringent BG control without frequent hypoglycemia or high glycemic variability was achieved by using CGGM. Future studies are needed to assess the practicality of using CGGM in a multi-center setting in addition to measurement of various inflammatory markers. The ultimate goal of these studies will be to perform a larger randomized controlled trial to evaluate the impact of improved glycemic control on transplant outcome.
AFFILIATIONS
- Internal Medicine, University of Washington, Seattle, WA, USA.
- Clinical Research Division, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
- UPMC Cancer Center, Pittsburgh, PA, USA.
- Massachusetts General Hospital Cancer Center, Boston, MA, USA.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Preiser JC, Devos P. Clinical experience with tight glucose control by intensive insulin therapy. Crit Care Med 2007; 35: S503-S507.
- van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001; 345: 1359-1367.
- Vanhorebeek I, Langouche L, Van den Berghe G. Tight blood glucose control with insulin in the ICU: facts and controversies. Chest 2007; 132: 268-278.
- Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med 2007; 35: 2262-2267.
- Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I et al. Intensive insulin therapy in the medical ICU. New Engl J Med 2006; 354: 449-461.
- Malmberg K, Ryden L, Wedel H, Birkeland K, Bootsma A, Dickstein K et al. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J 2005; 26: 650-661.
- Mehta SR, Yusuf S, Diaz R, Zhu J, Pais P, Xavier D et al. Effect of glucose-insulin- potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: the CREATE-ECLA randomized controlled trial. JAMA 2005; 293: 437-446.
- Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358: 125-139.
- Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc 2004; 79: 992-1000.
- Preiser JC, Devos P. Current status of tight blood sugar control. Curr Infect Dis Rep 2008; 10: 377-382.
- Gunst J, Van den Berghe G. Blood glucose control in the intensive care unit: benefits and risks. Semin Dial 2010; 23: 157-162.
- Umpierrez G, Cardona S, Pasquel F, Jacobs S, Peng L, Unigwe M et al. Randomized controlled trial of intensive versus conservative glucose control in patients undergoing coronary artery bypass graft surgery: GLUCO-CABG trial. Diabetes Care 2015; 38: 1665-1672.
- Van den Berghe G, Wilmer A, Milants I, Wouters PJ, Bouckaert B, Bruyninckx F et al. Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm. Diabetes 2006; 55: 3151-3159.
- Scalea TM, Bochicchio GV, Bochicchio KM, Johnson SB, Joshi M, Pyle A. Tight glycemic control in critically injured trauma patients. Ann Surg 2007; 246: 605-610.
- Bhatia A, Cadman B, Mackenzie I. Hypoglycemia and cardiac arrest in a critically ill patient on strict glycemic control. Anesth Analg 2006; 102: 549-551.
- Vriesendorp TM, DeVries JH, van Santen S, Moeniralam HS, de Jonge E, Roos YB et al. Evaluation of short-term consequences of hypoglycemia in an intensive care unit. Crit Care Med 2006; 34: 2714-2718.
- Krinsley JS, Jones RL. Cost analysis of intensive glycemic control in critically ill adult patients. Chest 2006; 129: 644-650.
- Egi M, Bellomo R, Stachowski E, French CJ, Hart G. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology 2006; 105: 244-252.
- Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med 2008; 36: 3008-3013.
- Ali NA, O’Brien JM Jr., Dungan K, Phillips G, Marsh CB, Lemeshow S et al. Glucose variability and mortality in patients with sepsis. Crit Care Med 2008; 36: 2316-2321.
- Fahy BG, Sheehy AM, Coursin DB. Glucose control in the intensive care unit. Crit Care Med 2009; 37: 1769–1776.
- Kurosawa S, Yakushijin K, Yamaguchi T, Atsuta Y, Nagamura-Inoue T, Akiyama H et al. Changes in incidence and causes of non-relapse mortality after allogeneic hematopoietic cell transplantation in patients with acute leukemia/myelodys-plastic syndrome: an analysis of the Japan Transplant Outcome Registry. Bone Marrow Transplant 2013; 48: 529-536.
- Kurosawa S, Yakushijin K, Yamaguchi T, Atsuta Y, Nagamura-Inoue T, Akiyama H et al. Recent decrease in non-relapse mortality due to GVHD and infection after allogeneic hematopoietic cell transplantation in non-remission acute leukemia. Bone Marrow Transplant 2013; 48: 1198-1204.
- Hammer MJ, Casper C, Gooley TA, O’Donnell PV, Boeckh M, Hirsch IB. The contribution of malglycemia to mortality among allogeneic hematopoietic cell transplant recipients. Biol Blood Marrow Transplant 2009; 15: 344-351.
- Fuji S, Kim SW, Mori S, Fukuda T, Kamiya S, Yamasaki S et al. Hyperglycemia during the neutropenic period is associated with a poor outcome in patients undergoing myeloablative allogeneic hematopoietic stem cell transplantation. Transplantation 2007; 84: 814-820.
- Gebremedhin E, Behrendt CE, Nakamura R, Parker P, Salehian B. Severe hyperglycemia immediately after allogeneic hematopoietic stem-cell transplan- tation is predictive of acute graft-versus-host disease. Inflammation 2013; 36: 177-185.
- Fuji S, Kim SW, Mori S, Kamiya S, Yoshimura K, Yokoyama H et al. Intensive glucose control after allogeneic hematopoietic stem cell transplantation: a retrospective matched-cohort study. Bone Marrow Transplant 2009; 44: 105-111.
- Davidson PC, Steed RD, Bode BW. Glucommander: a computer-directed intravenous insulin system shown to be safe, simple, and effective in 120,618h of operation. Diabetes Care 2005; 28: 2418-2423.
- Core Team R. R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria, 2015. URL https://www.R-project.org/.
- Wickham H. ggplot2: elegant graphics for data analysis. Springer: New York, NY, USA, 2009.
- Ouattara A, Lecomte P, Le Manach Y, Landi M, Jacqueminet S, Platonov I et al. Poor intraoperative blood glucose control is associated with a worsened hospital outcome after cardiac surgery in diabetic patients. Anesthesiology 2005; 103: 687-694.
- Williams LS, Rotich J, Qi R, Fineberg N, Espay A, Bruno A et al. Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke. Neurology 2002; 59: 67-71.
- Furnary AP, Wu Y. Clinical effects of hyperglycemia in the cardiac surgery population: the Portland Diabetic Project. Endocr Pract 2006; 12: 22-26.
- Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA 2008; 300: 933-944.
- Investigators N-SS, Finfer S, Chittock DR, Su SY, Blair D, Foster D et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360: 1283-1297.
- Kramer AH, Roberts DJ, Zygun DA. Optimal glycemic control in neurocritical care patients: a systematic review and meta-analysis. Crit Care 2012; 16: R203.
- Sheean PM, Freels SA, Helton WS, Braunschweig CA. Adverse clinical consequences of hyperglycemia from total parenteral nutrition exposure during hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2006; 12: 656-664.
- Investigators N-SS, Finfer S, Liu B, Chittock DR, Norton R, Myburgh JA et al. Hypoglycemia and risk of death in critically ill patients. N Engl J Med 2012; 367: 1108-1118.
- Antin JH. Acute graft-versus-host disease: inflammation run amok? J Clin Invest 2001; 107: 1497-1498.
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006; 116: 1793-1801.
- Pidala J, Kim J, Kharfan-Dabaja MA, Nishihori T, Field T, Perkins J et al. Dysglycemia following glucocorticoid therapy for acute graft-versus-host disease adversely affects transplantation outcomes. Biol Blood Marrow Transplant 2011; 17: 239-248.
- Golovchenko I, Goalstone ML, Watson P, Brownlee M, Draznin B. Hyperinsulinemia enhances transcriptional activity of nuclear factor-kappaB induced by angiotensin II, hyperglycemia, and advanced glycosylation end products in vascular smooth muscle cells. Circ Res 2000; 87: 746-752.
- Dandona P, Aljada A, Mohanty P, Ghanim H, Hamouda W, Assian E et al. Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab 2001; 86: 3257-3265.
- Fuji S, Einsele H, Savani BN, Kapp M. Systematic nutritional support in allogeneic hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant 2015; 21: 1707-1713.
- Avanzini F, Marelli G, Saltafossi D, Longhi C, Carbone S, Carlino L et al. Effectiveness, safety and feasibility of an evidence-based insulin infusion protocol targeting moderate glycaemic control in intensive cardiac care units. Eur Heart J Acute Cardiovasc Care 2015.
- Boutin JM, Gauthier L. Insulin infusion therapy in critically ill patients. Can J Diabetes 2014; 38: 144-150.
- Giani E, Scaramuzza AE, Zuccotti GV. Impact of new technologies on Diabetes Care. World J Diabetes 2015; 6: 999-1004.
- Scaramuzza AE, Zuccotti GV. Modern clinical management helps reducing the impact of type 1 diabetes in children. Pharmacol Res 2015; 98: 16-21.
- Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study G, Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008; 359: 1464-1476.
- Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long- term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care 2012; 35: 32138.
- Barassi A, Umbrello M, Ghilardi F, Damele CA, Massaccesi L, Iapichino G et al. Evaluation of the performance of a new OptiScanner 5000 system for an intermittent glucose monitoring. Clin Chim Acta 2015; 438: 252-254.